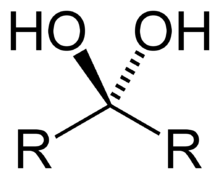
Geminal diol
A geminal diol (or gem-diol for short) is any organic compound having two hydroxyl functional groups (-OH) bound to the same carbon atom.
The simplest geminal diol is methanediol CH4O2 or H2C(OH)2. Other examples are dihydroxymalonic acid HOOC-C(OH)2-COOH and decahydroxycyclopentane (C(OH)2)5.
Geminal diols are a subclass of the diols, which in turn are a special class of alcohols.
The two hydroxyls in a geminal diol are easily converted to a carbonyl or keto group C=O by loss of one water molecule, thus turning the diol into a ketone. Conversely, ketones tend to combine with water to form the corresponding geminal diols. The equilibrium in water solution may be shifted towards either compound; for example, the equilibrium constant for the conversion of acetone (H3C)2C=O to propane-2,2-diol (H3C)2C(OH)2 is about 10−3,[1] while that of formaldehyde H2C=O to methanediol H2C(OH)2 is 10+3,[2] and that of hexafluoroacetone (F3C)2C=O to hexafluoropropane-2,2-diol (F3C)2C(OH)2 is about 10+6. In some cases, such as decahydroxycyclopentane and dodecahydroxycyclohexane, the geminal diol is stable while the ketone is not.
See also
References
- ↑ Peter Taylor (2002), Mechanism and synthesis, Book 10 of Molecular world. Open University, Royal Society of Chemistry; ISBN 0-85404-695-X. 368 pages.
- ↑ Eric V. Anslyn, Dennis A. Dougherty (2006), Modern physical organic chemistry. University Science Books. ISBN 1-891389-31-9. 1095 pages