Ionic liquid
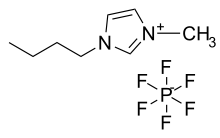

An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions and short-lived ion pairs. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. [1][2][3] They are known as "solvents of the future" as well as "designer solvents".
Ionic liquids are described as having many potential applications. They are powerful solvents and electrically conducting fluids (electrolytes). Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure.
Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at 801 °C (1,474 °F) into a liquid that consists largely of sodium cations (Na+
) and chloride anions (Cl−
). Conversely, when an ionic liquid is cooled, it often forms an ionic solid—which may be either crystalline or glassy.
The ionic bond is usually stronger than the Van der Waals forces between the molecules of ordinary liquids. For that reason, common salts tend to melt at higher temperatures than other solid molecules. Some salts are liquid at or below room temperature. Examples include compounds based on the 1-Ethyl-3-methylimidazolium (EMIM) cation and include: EMIM:Cl, EMIM dicyanamide, (C
2H
5)(CH
3)C
3H
3N+
2·N(CN)−
2, that melts at −21 °C (−6 °F);[4] and 1-butyl-3,5-dimethylpyridinium bromide which becomes a glass below −24 °C (−11 °F).[5]
Low-temperature ionic liquids can be compared to ionic solutions, liquids that contain both ions and neutral molecules, and in particular to the so-called deep eutectic solvents, mixtures of ionic and non-ionic solid substances which have much lower melting points than the pure compounds. Certain mixtures of nitrate salts can have melting points below 100 °C.[6]
The term "ionic liquid" in the general sense was used as early as 1943.[7]
Tawny crazy ants (Nylanderia fulva) exude an ionic liquid when they groom.[8]
History
The discovery date of the "first" ionic liquid is disputed, along with the identity of its discoverer. Ethanolammonium nitrate (m.p. 52–55 °C) was reported in 1888 by S. Gabriel and J. Weiner.[9] One of the earliest truly room temperature ionic liquids was ethylammonium nitrate (C
2H
5)NH+
3·NO−
3 (m.p. 12 °C), reported in 1914 by Paul Walden.[10] In the 1970s and 1980s, ionic liquids based on alkyl-substituted imidazolium and pyridinium cations, with halide or tetrahalogenoaluminate anions, were developed as potential electrolytes in batteries.[11][12]
For the imidazolium halogenoaluminate salts, their physical properties—such as viscosity, melting point, and acidity—could be adjusted by changing the alkyl substituents and the imidazolium/pyridinium and halide/halogenoaluminate ratios.[13] Two major drawbacks for some applications were moisture sensitivity and acidity/basicity. In 1992, Wilkes and Zawarotko obtained ionic liquids with 'neutral' weakly coordinating anions such as hexafluorophosphate (PF−
6) and tetrafluoroborate (BF−
4), allowing a much wider range of applications.[14]
Although many classical IL's are hexafluorophosphate and tetrafluoroborate salts, bistriflimide [(CF
3SO
2)
2N]−
are also popular.
Characteristics
Ionic liquids are often moderate to poor conductors of electricity, non-ionizing (e.g., non-polar), highly viscous and frequently exhibit low vapor pressure. Their other properties are diverse: many have low combustibility, are thermally stable, with wide liquid regions, and favorable solvating properties for a range of polar and non-polar compounds. Many classes of chemical reactions, such as Diels-Alder reactions and Friedel-Crafts reactions, can be performed using ionic liquids as solvents. IL's can serve as solvents for biocatalysis.[15] The miscibility of ionic liquids with water or organic solvents varies with side chain lengths on the cation and with choice of anion. They can be functionalized to act as acids, bases, or ligands, and are precursors salts in the preparation of stable carbenes. Because of their distinctive properties, ionic liquids are attracting increasing attention in many fields, including organic chemistry, electrochemistry, catalysis, physical chemistry, and engineering; see for instance magnetic ionic liquid.
Despite their extremely low vapor pressures (≈10−10 Pa at 25˚C), some ionic liquids can be distilled under vacuum conditions at temperatures near 300 °C.[16] In the original work by Martyn Earle, et al., the authors wrongly concluded that the vapor was made up of individual, separated ions,[17] but was later proven that the vapors formed consisted of ion-pairs.[18] Some ionic liquids (such as 1-butyl-3-methylimidazolium nitrate) generate flammable gases on thermal decomposition. Thermal stability and melting point depend on the liquid's components. Thermal stability of various RTILs (Room Temperature Ionic Liquid) are available. The thermal stability of a task-specific ionic liquid, protonated betaine bis(trifluoromethanesulfonyl)imide is of about 534 K (502 °F) and N-Butyl-N-Methyl pyrrolidinium bis(trifluoromethanesulfonyl)imide was thermally stable up to 640 K.[19] The upper limits of thermal stability of ionic liquids reported in the literature are usually based upon fast (about 10 K/min) TGA scans, and they do not imply long-term (several hours) thermal stability of ionic liquids, which is limited to less than 500 K for most ionic liquids.[20]
The solubility of different species in imidazolium ionic liquids depends mainly on polarity and hydrogen bonding ability. Saturated aliphatic compounds are generally only sparingly soluble in ionic liquids, whereas olefins show somewhat greater solubility, and aldehydes can be completely miscible. This can be exploited in biphasic catalysis, such as hydrogenation and hydrocarbonylation processes, allowing for relatively easy separation of products and/or unreacted substrate(s). Gas solubility follows the same trend, with carbon dioxide gas showing exceptional solubility in many ionic liquids. Carbon monoxide is less soluble in ionic liquids than in many popular organic solvents, and hydrogen is only slightly soluble (similar to the solubility in water) and may vary relatively little between the more common ionic liquids. Different analytical techniques have yielded somewhat different absolute solubility values.
Room temperature varieties
Room temperature ionic liquids consist of bulky and asymmetric organic cations such as 1-alkyl-3-methylimidazolium, 1-alkylpyridinium, N-methyl-N-alkylpyrrolidinium and ammonium ions. Phosphonium cations are less common, but offer some advantageous properties.[21][22] A wide range of anions are employed, ranging from simple halides, which generally suffer high melting points, to inorganic anions such as tetrafluoroborate and hexafluorophosphate, and to large organic anions like bistriflimide, triflate or tosylate. There are also many potential uses of ionic liquids with simple non-halogenated organic anions such as formate, alkylsulfate, alkylphosphate or glycolate. The melting point of 1-butyl-3-methylimidazolium tetrafluoroborate with an imidazole skeleton is about −80 °C (−112 °F) and it is a colorless liquid with high viscosity at room temperature. If a highly asymmetric cation is combined with a highly asymmetric anion, formed ionic liquid may not freeze down to very low temperatures (down to -150 °C) and the glass transition temperature was detected below -100 °C in the case of ionic liquids with N-methyl-N-alkylpyrrolidinium cations and fluorosulfonyl-trifluoromethanesulfonylimide (FTFSI).[23]
In many synthetic processes using transition metal catalysts, metal nanoparticles play an important role as the actual catalyst or as a catalyst reservoir. ILs are an appealing medium for the formation and stabilization of catalytically active transition metal nanoparticles. More importantly, ILs can be made that incorporate coordinating groups,[24] for example, with nitrile groups on either the cation or anion (CN-IL). In various C-C coupling reactions catalyzed by a palladium catalyst, it has been found that palladium nanoparticles are better stabilized in CN-IL compared to non-functionalized ionic liquids; thus enhanced catalytic activity and recyclability are realized.[25]
Low temperature varieties
Low temperature ionic liquids (below 130 K) have been proposed as the fluid base for an extremely large diameter spinning liquid mirror telescope to be based on the Earth's moon.[26] Low temperature is advantageous in imaging long wave infrared light which is the form of light (extremely red-shifted) that arrives from the most distant parts of the visible universe. Such a liquid base would be covered by a thin metallic film that forms the reflective surface. Low volatility is important in lunar vacuum conditions.
Commercial applications
A liquid tetraalkylphosphonium iodide is a solvent for tributyltin iodide, which functions as a catalyst to rearrange the monoepoxide of butadiene. This process was commercialized as a route to 2,5-dihydrofuran, but later discontinued[27]
Potential applications
Gas handling
ILs have been considered for a variety of industrial applications. Attractive in gas storage and handling applications, are their low vapor pressure, thermal stability, and solvation for a wide variety of compounds and gases. Air Products uses ILs instead of pressurized cylinders as a transport medium for reactive gases such as trifluoroborane, phosphine and arsine. The gases are dissolved in the liquids at or below atmospheric pressure and are easily withdrawn from the containers by applying a vacuum. Gas manufacturer Linde exploits the low solubility of hydrogen in ILs to compress the gas up to 450 bar in filling stations by using an ionic liquid piston compressor,
Pharmaceuticals
Recognizing that approximately 50% of commercial pharmaceuticals are organic salts, ionic liquid forms of a number of pharmaceuticals have been investigated. Combining a pharmaceutically active cation with a pharmaceutically active anion leads to a Dual Active ionic liquid in which the actions of two drugs are combined.[28][29]
ILs can extract specific compounds from plants for pharmaceutical, nutritional and cosmetic applications, such as the antimalarial drug artemisinin from the plant Artemisia annua.[30]
Cellulose processing
The dissolution of cellulose by ILs has attracted interest.[31] A patent application from 1930 showed that 1-alkylpyridinium chlorides dissolve cellulose.[32] Following in the footsteps of the lyocell process, which uses hydrated N-Methylmorpholine N-oxide, as a non-aqueous solvent for the dissolution of the pulp and paper. The dissolution of cellulose–based materials like tissue paper waste, generated in chemical industries and at research laboratories, in room temperature IL 1-butyl-3-methylimidazolium chloride, bmimCl and the recovery of valuable compounds by electrodeposition from this cellulose matrix was studied.[33] The "valorization" of cellulose, i.e. its conversion to more valuable chemicals, has been achieved by the use of ionic liquids. Representative products are glucose esters, sorbitol, and alkylgycosides.[34] IL 1-butyl-3-methylimidazolium chloride dissolves freeze dried banana pulp and with an additional 15% DMSO, lends itself to Carbon-13 NMR analysis. In this way the entire complex of starch, sucrose, glucose, and fructose can be monitored as a function of banana ripening.[35]
ILs are effective for extracting chemicals potentially relevant to biodiesel, ethanol, and other biofuels from algae.[36]
Gas handling
The IL 1-butyl-3-methylimidazolium chloride has been investigated for separating hydrogen from ammonia borane.[37]
ILs and amines have been investigated for capture carbon dioxide CO
2 and purify natural gas.[38][39][40]
Nuclear fuel reprocessing
The IL 1-butyl-3-methylimidazolium chloride has been investigated as a non-aqueous electrolyte media for the recovery of uranium and other metals from spent nuclear fuel and other sources.[41][42][43] Protonated betaine bis(trifluoromethanesulfonyl) imide has been investigated as a solvent for uranium oxides.[44] Ionic liquids, N-butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide and N-methyl-N-propylpiperidinium bis(trifluoromethylsulfonyl)imide, have been investigated for the electrodeposition of europium and uranium metals respectively.[45][46]
Solar thermal energy
ILs are potential heat transfer and storage media in solar thermal energy systems. Concentrating solar thermal facilities such as parabolic troughs and solar power towers focus the sun's energy onto a receiver which can generate temperatures of around 600 °C (1,112 °F). This heat can then generate electricity in a steam or other cycle. For buffering during cloudy periods or to enable generation overnight, energy can be stored by heating an intermediate fluid. Although nitrate salts have been the medium of choice since the early 1980s, they freeze at 220 °C (428 °F) and thus require heating to prevent solidification. Ionic liquids such as Cmim
4[BF
4] have more favorable liquid-phase temperature ranges (-75 to 459 °C) and could therefore be excellent liquid thermal storage media and heat transfer fluids.[47]
Waste recycling
ILs can aid the recycling of synthetic goods, plastics and metals. They offer the specificity required to separate similar compounds from each other, such as separating polymers in plastic waste streams. This has been achieved using lower temperature extraction processes than current approaches[48] and could help avoid incinerating plastics or dumping them in landfill.
Batteries
ILs can replace water as the electrolyte in metal-air batteries. ILs are attractive because of their low vapor pressure, increasing battery life by drying slower. Furthermore, ILs have an electrochemical window of up to six volts[49] (versus 1.23 for water) supporting more energy-dense metals. Energy densities from 900-1600 watt-hours per kilogram appear possible.[50]
A Metal-air battery draws oxygen through a porous ambient "air" electrode (-cathode) and produces water, hydrogen peroxide, or hydroxide anions depending on the nature oxygen reduction catalyst and electrolyte. These compounds store the electrons released by the oxidation of the anode.
ILs can act as dispersing agents in paints to enhance finish, appearance and drying properties.[51] ILs are used for dispersing nanomaterials at IOLITEC.
Carbon capture
Ionic liquids have been proposed as an absorbent in carbon capture. They have various advantages over traditional absorbents, such as the currently dominant amine-based technologies. 1-Butyl-3-methylimidazolium hexafluorophosphate is one example of a proposed CO2 absorbent.
Safety
Ionic liquids' low volatility effectively eliminates a major pathway for environmental release and contamination. However, this property is distinct from toxicity. Ionic liquids' aquatic toxicity is as severe as or more so than many current solvents.[52][53][54] Mortality isn't necessarily the most important metric for measuring impacts in aquatic environments, as sub-lethal concentrations change organisms' life histories in meaningful ways. Balancing VOC reductions against waterway spills (via waste ponds/streams, etc.) requires further research. Ionic liquids' substituent diversity simplify the process of identifying compounds that meet safety requirements.
Ultrasound can degrade solutions of imidazolium-based ionic liquids with hydrogen peroxide and acetic acid to relatively innocuous compounds.[55]
Despite low vapor pressure many ionic liquids are combustible and therefore require careful handling.[56] Brief exposure (5 to 7 seconds) to a flame torch can ignite some Ionic liquids. Complete combustion is possible for some Ionic liquids.[57]
See also
- MDynaMix software for ionic liquids simulations
- 1-Butyl-3-methylimidazolium hexafluorophosphate (BMIM-PF6) for an often encountered ionic liquid
- Trioctylmethylammonium bis(trifluoromethyl-sulfonyl)imide
- Aza-Baylis–Hillman reaction for the use of a chiral ionic liquid in asymmetric synthesis.
- Ionic liquids in carbon capture
- NanoFlowcell which uses ionic liquid in its car batteries
References
- ↑ Thomas Welton (1999). "Room-Temperature Ionic Liquids". Chem. Rev. 99: 2071–2084. doi:10.1021/cr980032t. PMID 11849019.
- ↑ F. Endres; S. Zein El Abedin (2006). "Air and water stable ionic liquids in physical chemistry". Phys. Chem. Chem. Phys. 8 (18): 2101–16. Bibcode:2006PCCP....8.2101E. doi:10.1039/b600519p. PMID 16751868.
- ↑ Freemantle, Michael (2009). An Introduction to Ionic Liquids. Royal Society of Chemistry. ISBN 978-1-84755-161-0.
- ↑ D. R. MacFarlane; J. Golding; S. Forsyth; M. Forsyth & G. B. Deacon (2001). "Low viscosity ionic liquids based on organic salts of the dicyanamide anion". Chem. Commun. (16): 1430–1431. doi:10.1039/b103064g.
- ↑ J. M Crosthwaite; M. J. Muldoon; J. K. Dixon; J. L. Anderson & J. F. Brennecke (2005). "Phase transition and decomposition temperatures, heat capacities and viscosities of pyridinium ionic liquids". J. Chem. Thermodyn. 37 (6): 559–568. doi:10.1016/j.jct.2005.03.013.
- ↑ Mixture of nitrate salts with m.p. below 100 deg C
- ↑ R. M. Barrer (1943). "The Viscosity of Pure Liquids. II. Polymerised Ionic Melts". Trans. Faraday Soc. 39: 59–67. doi:10.1039/tf9433900059.
- ↑ "On the Formation of a Protic Ionic Liquid in Nature". Angewandte Chemie International Edition. 53 (44): 11762–11765. 2014. doi:10.1002/anie.201404402.
- ↑ S. Gabriel; J. Weiner (1888). "Ueber einige Abkömmlinge des Propylamins". Ber. 21 (2): 2669–2679. doi:10.1002/cber.18880210288.
- ↑ Paul Walden (1914), Bull. Acad. Sci. St. Petersburg, pages 405-422.
- ↑ H. L. Chum; V. R. Koch; L. L. Miller; R. A. Osteryoung (1975). "Electrochemical scrutiny of organometallic iron complexes and hexamethylbenzene in a room temperature molten salt". J. Am. Chem. Soc. 97 (11): 3264–3265. doi:10.1021/ja00844a081.
- ↑ J. S. Wilkes; J. A. Levisky; R. A. Wilson; C. L. Hussey (1982). "Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis". Inorg. Chem. 21 (3): 1263–1264. doi:10.1021/ic00133a078.
- ↑ R. J. Gale; R. A. Osteryoung (1979). "Potentiometric investigation of dialuminium heptachloride formation in aluminum chloride-1-butylpyridinium chloride mixtures". Inorganic Chemistry. 18 (6): 1603–1605. doi:10.1021/ic50196a044.
- ↑ Wilkes J. S.; Zaworotko M. J. Chemical Communications. 1992: 965–967. Missing or empty
|title=(help) - ↑ Adam J. Walker & Neil C. Bruce (2004). "Cofactor-dependent enzyme catalysis in functionalized ionic solvents". Chemical Communications. 2004 (22): 2570–1. doi:10.1039/b410467f. PMID 15543284.
- ↑ Martyn J. Earle; José M.S.S. Esperança; Manuela A. Gilea; José N. Canongia Lopes; Luís P.N. Rebelo; Joseph W. Magee; Kenneth R. Seddon & Jason A. Widegren (2006). "The distillation and volatility of ionic liquids". Nature. 439 (7078): 831–4. Bibcode:2006Natur.439..831E. doi:10.1038/nature04451. PMID 16482154.
- ↑ Peter Wasserscheid (2006). "Volatile times for ionic liquids". Nature. 439 (7078): 797. Bibcode:2006Natur.439..797W. doi:10.1038/439797a. PMID 16482141.
- ↑ James P. Armstrong; Christopher Hurst; Robert G. Jones; Peter Licence; Kevin R. J. Lovelock; Christopher J. Satterley & Ignacio J. Villar-Garcia (2007). "Vapourisation of ionic liquids". Physical Chemistry Chemical Physics. 9 (8): 982–90. Bibcode:2007PCCP....9..982A. doi:10.1039/b615137j. PMID 17301888.
- ↑ Ch. Jagadeeswara Rao, R. Venkata krishnan, K. A. Venkatesan, K. Nagarajan, 332 - 334, Feb. 4-6, Sixteenth national symposium on thermal analysis(Thermans 2008)
- ↑ Marek Kosmulski; Jan Gustafsson & Jarl B. Rosenholm (2004). "Thermal stability of low temperature ionic liquids revisited". Thermochimica Acta. 412: 47–53. doi:10.1016/j.tca.2003.08.022.
- ↑ K. J. Fraser; D. R. MacFarlane (2009). "Phosphonium-Based Ionic Liquids: An Overview". Aust J. Chem. 62: 309–321. doi:10.1071/ch08558., https://www.researchgate.net/publication/225089857_Phosphonium-Based_Ionic_Liquids_An_Overview
- ↑ Jiangshui Luo; Olaf Conrad & Ivo F. J. Vankelecom (2012). "Physicochemical properties of phosphonium-based and ammonium-based protic ionic liquids". Journal of Materials Chemistry. 22: 20574–20579. doi:10.1039/C2JM34359B.
- ↑ Reiter, Jakub (2 Sep 2012). "Fluorosulfonyl-(trifluoromethanesulfonyl)imide ionic liquids with enhanced asymmetry". Physical Chemistry Chemical Physics. 15: 2565–2571. Bibcode:2013PCCP...15.2565R. doi:10.1039/c2cp43066e. Retrieved 2012-12-12.
- ↑ X. Li; D. Zhao; Z. Fei; L. Wang (2006). "Applications of Functionalized Ionic Liquids". Science in China: B. 35 (5): 181. doi:10.1007/s11426-006-2020-y.
- ↑ Zhao, D.; Fei, Z.; Geldbach, T. J.; Scopelliti, R.; Dyson, P. J. (2004). "Nitrile-Functionalized Pyridinium Ionic Liquids: Synthesis, Characterization, and Their Application in Carbon-Carbon Coupling Reactions". J. Am. Chem. Soc. 126 (48): 15876–82. doi:10.1021/ja0463482. PMID 15571412.
- ↑ E. F. Borra; O. Seddiki; R. Angel; D. Eisenstein; P. Hickson; K. R. Seddon & S. P. Worden (2007). "Deposition of metal films on an ionic liquid as a basis for a lunar telescope". Nature. 447 (7147): 979–981. Bibcode:2007Natur.447..979B. doi:10.1038/nature05909. PMID 17581579.
- ↑ G. Wytze Meindersma, Matthias Maase and André B. Haan "Ionic Liquids" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.l14_l01
- ↑ J. Stoimenovski; D. R. MacFarlane; K. Bica; R. D. Rogers (2010). "Crystalline vs. Ionic Liquid Salt Forms of Active Pharmaceutical Ingredients: A Position Paper". Pharmaceutical Research. 27: 521–526. doi:10.1007/s11095-009-0030-0.
- ↑ Frank Postleb; Danuta Stefanik; Harald Seifert & Ralf Giernoth (2013). "BIOnic Liquids: Imidazolium-based Ionic Liquids with Antimicrobial Activity". Zeitschrift für Naturforschung B. 68b: 1123 – 1128. doi:10.5560/ZNB.2013-3150.
- ↑ A.Lapkin; P.K. Plucinski; M. Cutler (2006). "Comparative assessment of technologies for extraction of artemisinin". Journal of Natural Products. 11 (69): 1653–1664. doi:10.1021/np060375j. PMID 17125242.
- ↑ Richard P. Swatloski; Scott K. Spear; John D. Holbrey & Robin D. Rogers (2002). "Dissolution of Cellose with Ionic Liquids". Journal of the American Chemical Society. 124/18 (18): 4974–4975. doi:10.1021/ja025790m.
- ↑ Charles Graenacher, Manufacture and Application of New Cellulose Solutions and Cellulose Derivatives Produced therefrom, US 1934/1943176.
- ↑ Ch. Jagadeeswara Raoa; K.A. Venkatesana; K. Nagarajana; T.G. Srinivasan & P.R. Vasudeva Rao (2007). "Treatment of tissue paper containing radioactive waste and electrochemical recovery of valuables using ionic liquids". Electrochimica Acta. 53 (4): 1911–1919. doi:10.1016/j.electacta.2007.08.043.
- ↑ Ignatyev, Igor; Charlie Van Doorslaer; Pascal G.N. Mertens; Koen Binnemans; Dirk. E. de Vos (2011). "Synthesis of glucose esters from cellulose in ionic liquids". Holzforschung. 66 (4): 417–425. doi:10.1515/hf.2011.161.
- ↑ Fort, D.A, Swatloski, R.P., Moyna, P., Rogers, R.D., Moyna, G. "Use of ionic liquids in the study of fruit ripening by high-resolution 13C NMR spectroscopy: ‘green’ solvents meet green bananas" Chem. Commun. 2006, 714. doi:10.1039/B515177P
- ↑ R. E. Teixeira (2012). "Energy-efficient extraction of fuel and chemical feedstocks from algae". Green Chemistry. 14 (2): 419–427. doi:10.1039/C2GC16225C.
- ↑ A. Karkamkar; C. Aardahl; T. Autrey (2007). "Recent Developments on Hydrogen Release from Ammonia Borane" (PDF). Material Matters. 2 (2): 6–9. Archived from the original (PDF) on July 18, 2009.
- ↑ C&E News
- ↑ Barghi S.H.; Adibi M.; Rashtchian D. (2010). "An experimental study on permeability, diffusivity, and selectivity of CO2 and CH4 through [bmim][PF6] ionic liquid supported on an alumina membrane: Investigation of temperature fluctuations effects". Journal of Membrane Science. 362: 346–352. doi:10.1016/j.memsci.2010.06.047.
- ↑ Mota-Martinez M. T.; Althuluth M.; Berrouk A.; Kroon M.C.; Peters Cor J. (2014). "High pressure phase equilibria of binary mixtures of light hydrocarbons in the ionic liquid 1-hexyl-3-methylimidazolium tetracyanoborate". Fluid Phase Equilibria. 362: 96–101. doi:10.1016/j.fluid.2013.09.015.
- ↑ P. Giridhar, K.A. Venkatesan, T.G. Srinivasan and P.R. Vasudeva Rao (2007), Electrochemical behavior of uranium(VI) in 1-butyl-3-methylimidazolium chloride and thermal characterization of uranium oxide deposit, Electrochimica Acta, Volume 52, Issue 9, Pages 3006-3012,
- ↑ Jayakumar M.; Venkatesan K.A.; Srinivasan T.G. (2007). "Electrochemical behavior of fission palladium in 1-butyl-3-methylimidazolium chloride". Electrochimica Acta. 52 (24): 7121–7127. doi:10.1016/j.electacta.2007.05.049.
- ↑ Jayakumar M.; Venkatesan K.A.; Srinivasan T.G.; Rao P.R. Vasudeva. "Extraction-Electrodeposition (EX-EL) process for the recovery of fission palladium from high-level liquid waste". J. Applied Electrochem. 39: 1955–1962. doi:10.1007/s10800-009-9905-3.
- ↑ Ch , Rao Jagadeeswara, Venkatesan K.A., Nagarajan K., Srinivasan T.G. (2008). "Dissolution of uranium oxides and electrochemical behavior of U(VI) in task specific ionic liquid". Radiochimica acta. 96 (7): 403409. doi:10.1524/ract.2008.1508.
- ↑ Ch. Jagadeeswara Rao, K.A. Venkatesan, K. Nagarajan, T.G. Srinivasan and P. R. Vasudeva Rao, Electrodeposition of metallic uranium at near ambient conditions from room temperature ionic liquid, Journal of Nuclear Materials, 408 (2011) 25–29.
- ↑ Ch , Rao Jagadeeswara, Venkatesan K.A., Nagarajan K., Srinivasan T.G., Rao P. R. Vasudeva. "Electrochemical behavior of europium (III) in N-butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide". Electrochimica Acta. 54 (20): 4718–4725.
- ↑ Banqui Wu; Ramana G. Reddy & Robin D. Rogers (2001). "Novel ionic liquid thermal storage for solar thermal electric power systems". International Solar Energy Conference: 445–451.
- ↑ Archived March 12, 2009, at the Wayback Machine.
- ↑ Michel Armand; Frank Endres; Douglas R. MacFarlane; Hiroyuki Ohno & Bruno Scrosati (2009). "Ionic-liquid materials for the electrochemical challenges of the future". Nature Materials. 8 (8): 621–629. Bibcode:2009NatMa...8..621A. doi:10.1038/nmat2448. PMID 19629083.
- ↑ "Betting on a Metal-Air Battery Breakthrough". Technology Review. November 5, 2009. Retrieved November 7, 2009.
- ↑ Examples are the TEGO brand dispersers by Evonik, used in their Pliolite brand paints.
- ↑ C Pretti; C Chiappe; D Pieraccini; M Gregori; F Abramo; G Monni & L Intorre (2006). "Acute toxicity of ionic liquids to the zebrafish (Danio rerio)". Green Chem. 8 (3): 238–240. doi:10.1039/b511554j.
- ↑ D. Zhao; Y. Liao & Z. Zhang (2007). "Toxicity of Ionic Liquids". CLEAN - Soil, Air, Water. 35 (1): 42–48. doi:10.1002/clen.200600015.
- ↑ J Ranke; S Stolte; R Störmann; J Arning & B Jastorff (2007). "Design of sustainable chemical products – the example of ionic liquids". Chem. Rev. 107 (6): 2183–2206. doi:10.1021/cr050942s. PMID 17564479.
- ↑ Xuehui Li; Jinggan Zhao; Qianhe Li; Lefu Wang & Shik Chi Tsang (2007). "Ultrasonic chemical oxidative degradations of 1,3-dialkylimidazolium ionic liquids and their mechanistic elucidations". Dalton Trans. (19): 1875. doi:10.1039/b618384k.
- ↑ Marcin Smiglak; W. Mathew Reichert; John D. Holbrey; John S. Wilkes; Luyi Sun; Joseph S. Thrasher; Kostyantyn Kirichenko; et al. (2006). "Combustible ionic liquids by design: is laboratory safety another ionic liquid myth?". Chemical Communications. 2006 (24): 2554–2556. doi:10.1039/b602086k. PMID 16779475.
- ↑ Uwe Schaller; Thomas Keicher; Volker Weiser; Horst Krause; Stefan Schlechtriem (2010-07-10). "Synthesis, Characterization and Combustion of Triazolium Based Salts" (pdf). pp. 1–23. Retrieved 2016-03-02.
External links
- Ionic Liquids Biological Effects Database, free database on toxicology and ecotoxicology of ionic liquids
- Corresponding states for ionic fluids