Iron-based superconductor
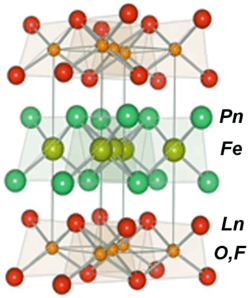
Iron-based superconductors (FeSC) are iron-containing chemical compounds whose superconducting properties were discovered in 2006.[2][3] In 2008, led by recently discovered iron pnictide compounds (originally known as oxypnictides), they were in the first stages of experimentation and implementation.[4] (Previously most high-temperature superconductors were cuprates and being based on layers of copper and oxygen sandwiched between other substances (La, Ba, Hg)).
This new type of superconductors is based instead on conducting layers of iron and a pnictide (chemical elements in group 15 of the periodic table, here typically arsenic (As) and phosphorus (P)) and seems to show promise as the next generation of high temperature superconductors.[5]
Much of the interest is because the new compounds are very different from the cuprates and may help lead to a theory of non-BCS-theory superconductivity.
More recently these have been called the ferropnictides. The first ones found belong to the group of oxypnictides. Some of the compounds have been known since 1995,[6] and their semiconductive properties have been known and patented since 2006.[7]
It has also been found that some iron chalcogens superconduct.[8] The undoped β-FeSe is the simplest iron-based superconductor but with the diverse properties.[9] It has a critical temperature (Tc) of 8 K at normal pressure, and 36.7 K under high pressure[10] and by means of intercalation. The combination of both intercalation and pressure results in re-emerging superconductivity at 48 (see [9] and references therein).
A subset of iron-based superconductors with properties similar to the oxypnictides, known as the 122 iron arsenides, attracted attention in 2008 due to their relative ease of synthesis.
The oxypnictides such as LaOFeAs are often referred to as the '1111' pnictides.
The crystalline material, known chemically as LaOFeAs, stacks iron and arsenic layers, where the electrons flow, between planes of lanthanum and oxygen. Replacing up to 11 percent of the oxygen with fluorine improved the compound — it became superconductive at 26 kelvin, the team reports in the March 19, 2008 Journal of the American Chemical Society. Subsequent research from other groups suggests that replacing the lanthanum in LaOFeAs with other rare earth elements such as cerium, samarium, neodymium and praseodymium leads to superconductors that work at 52 kelvin.[5]
| Oxypnictide | Tc (K) |
|---|---|
| LaO0.89F0.11FeAs | 26[11] |
| LaO0.9F0.2FeAs | 28.5[12] |
| CeFeAsO0.84F0.16 | 41[11] |
| SmFeAsO0.9F0.1 | 43[11][13] |
| La0.5Y0.5FeAsO0.6 | 43.1[14] |
| NdFeAsO0.89F0.11 | 52[11] |
| PrFeAsO0.89F0.11 | 52[15] |
| ErFeAsO1–y | 45[16] |
| Al-32522 (CaAlOFeAs) | 30(As), 16.6 (P)[17] |
| Al-42622 (CaAlOFeAs) | 28.3(As), 17.2 (P)[18] |
| GdFeAsO0.85 | 53.5[19] |
| BaFe1.8Co0.2As2 | 25.3[20] |
| SmFeAsO~0.85 | 55[21] |
| Non-oxypnictide | Tc (K) |
|---|---|
| Ba0.6K0.4Fe2As2 | 38[22] |
| Ca0.6Na0.4Fe2As2 | 26[23] |
| CaFe0.9Co0.1AsF | 22[24] |
| Sr0.5Sm0.5FeAsF | 56[25] |
| LiFeAs | 18[26][27][28] |
| NaFeAs | 9–25[29][30] |
| FeSe | <27[31][32] |
Iron pnictide superconductors crystallize into the [FeAs] layered structure alternating with spacer or charge reservoir block.[11] The compounds can thus be classified into “1111” system RFeAsO (R: the rare earth element) including LaFeAsO,[3] SmFeAsO,[13] PrFeAsO,[21] etc.; “122” type BaFe2As2,[22] SrFe2As2[33] or CaFe2As2;[23] “111” type LiFeAs,[26][27][28] NaFeAs,[29][30][34] and LiFeP.[35] Doping or applied pressure will transform the compounds into superconductors.[11][36][37]
Compounds such as Sr2ScFePO3 discovered in 2009 are referred to as the '42622' family, as FePSr2ScO3.[38] Noteworthy is the synthesis of (Ca4Al2O6-y)(Fe2Pn2) (or Al-42622(Pn); Pn = As and P) using high-pressure synthesis technique. Al-42622(Pn) exhibit superconductivity for both Pn = As and P with the transition temperatures of 28.3 K and 17.1 K, respectively. The a-lattice parameters of Al-42622(Pn) (a = 3.713 Å and 3.692 Å for Pn = As and P, respectively) are smallest among the iron-pnictide superconductors. Correspondingly, Al-42622(As) has the smallest As-Fe-As bond angle (102.1°) and the largest As distance from the Fe planes (1.5 Å).[18] High-pressure technique also yields (Ca3Al2O5-y)(Fe2Pn2) (Pn = As and P), the first reported iron-based superconductors with the perovskite-based '32522' structure. The transition temperature (Tc) is 30.2 K for Pn = As and 16.6 K for Pn = P. The emergence of superconductivity is ascribed to the small tetragonal a-axis lattice constant of these materials. From these results, an empirical relationship was established between the a-axis lattice constant and Tc in iron-based superconductors.[17]
In 2009, it was shown that undoped iron pnictides had a magnetic quantum critical point deriving from competition between electronic localization and itinerancy.[39]
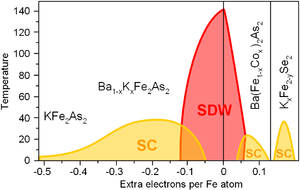
Phase diagrams
Similarly to superconducting cuprates, the properties of iron based superconductors change dramatically with doping. Parent compounds of FeSC are usually metals (unlike the cuprates) but, similarly to cuprates, are ordered antiferromagnetically that often termed as a spin-density wave (SDW). The superconductivity (SC) emerges upon either hole or electron doping. In general, the phase diagram is similar to the cuprates.
Superconductivity
Superconducting transition temperatures are listed in the tables (some at high pressure). BaFe1.8Co0.2As2 is predicted to have an upper critical field of 43 tesla from the measured coherence length of 2.8 nm.[20]
In 2011, Japanese scientists stumbled across a discovery which increased a metal compound's superconductivity by immersing iron-based compounds in hot alcoholic beverages such as red wine.[40][41][42] Earlier reports indicated that excess Fe is the cause of the bicollinear antiferromagnetic order and is not in favor of superconductivity. Further investigation revealed that weak acid has the ability to deintercalate the excess Fe from the interlayer sites. Therefore,weak acid annealing suppresses the antiferromagnetic correlation by deintercalating the excess Fe and, hence superconductivity is achieved.[43]
See also
- Andreev reflection
- Charge transfer complex
- Color superconductivity in quarks
- Composite Reaction Texturing
- Conventional superconductor
- covalent superconductors
- High-temperature superconductivity
- Homes's law
- Kondo effect
- Little-Parks effect
- Magnetic sail
- National Superconducting Cyclotron Laboratory
- Oxypnictide
- Proximity effect
- Room temperature superconductor
- Rutherford cable
- Spallation Neutron Source
- Superconducting RF
- Superconductor classification
- Superfluid film
- Technological applications of superconductivity
- Timeline of low-temperature technology
- Type-I superconductor
- Type-II superconductor
- Unconventional superconductor
References
- 1 2 Hosono, H.; Tanabe, K.; Takayama-Muromachi, E.; Kageyama, H.; Yamanaka, S.; Kumakura, H.; Nohara, M.; Hiramatsu, H.; Fujitsu, S. (2015). "Exploration of new superconductors and functional materials, and fabrication of superconducting tapes and wires of iron pnictides". Science and Technology of Advanced Materials. 16 (3): 033503. arXiv:1505.02240
 . Bibcode:2015STAdM..16c3503H. doi:10.1088/1468-6996/16/3/033503. PMC 5099821
. Bibcode:2015STAdM..16c3503H. doi:10.1088/1468-6996/16/3/033503. PMC 5099821 . PMID 27877784.
. PMID 27877784. - ↑ Kamihara, Yoichi; Hiramatsu, Hidenori; Hirano, Masahiro; Kawamura, Ryuto; Yanagi, Hiroshi; Kamiya, Toshio; Hosono, Hideo (2006). "Iron-Based Layered Superconductor: LaOFeP". J. Am. Chem. Soc. 128 (31): 10012–10013. doi:10.1021/ja063355c. PMID 16881620.
- 1 2 Kamihara, Yoichi; Watanabe, Takumi; Hirano, Masahiro; Hosono, Hideo (2008). "Iron-Based Layered Superconductor La[O1–xFx]FeAs (x = 0.05–0.12) with Tc = 26 K". Journal of the American Chemical Society. 130 (11): 3296–3297. doi:10.1021/ja800073m. PMID 18293989.
- ↑ Ozawa, T C; Kauzlarich, S M (2008). "Chemistry of layered d-metal pnictide oxides and their potential as candidates for new superconductors". Sci. Technol. Adv. Mater. 9 (3): 033003. arXiv:0808.1158
 . Bibcode:2008STAdM...9c3003O. doi:10.1088/1468-6996/9/3/033003.
. Bibcode:2008STAdM...9c3003O. doi:10.1088/1468-6996/9/3/033003. 
- 1 2 "Iron Exposed as High-Temperature Superconductor". Scientific American. June 2008
- ↑ Zimmer, Barbara I.; Jeitschko, Wolfgang; Albering, Jörg H.; Glaum, Robert; Reehuis, Manfred (1995). "The rate earth transition metal phosphide oxides LnFePO, LnRuPO and LnCoPO with ZrCuSiAs type structure". Journal of Alloys and Compounds. 229 (2): 238–242. doi:10.1016/0925-8388(95)01672-4.
- ↑ Hosono, H. et al. (2006) Magnetic semiconductor material European Patent Application EP1868215
- ↑ Johannes, Michelle (2008). "The iron age of superconductivity". Physics. 1: 28. Bibcode:2008PhyOJ...1...28J. doi:10.1103/Physics.1.28.
- 1 2 Yu. V. Pustovit and A. A. Kordyuk (2016). "Metamorphoses of electronic structure of FeSe-based superconductors (Review article)". Low. Temp. Phys. 42. arXiv:1608.07751
 .
. - ↑ Medvedev, S.; McQueen, T. M.; Troyan, I. A.; Palasyuk, T.; Eremets, M. I.; Cava, R. J.; Naghavi, S.; Casper, F.; Ksenofontov, V.; Wortmann, G.; Felser, C. (2009). "Electronic and Magnetic Phase Diagram of β-Fe1.01Se with superconductivity at 36.7 K under pressure". Nature Materials. 8 (8): 630–633. arXiv:0903.2143
 . Bibcode:2009NatMa...8..630M. doi:10.1038/nmat2491. PMID 19525948.
. Bibcode:2009NatMa...8..630M. doi:10.1038/nmat2491. PMID 19525948. - 1 2 3 4 5 6 Ishida, Kenji; Nakai, Yusuke; Hosono, Hideo (2009). "To What Extent Iron-Pnictide New Superconductors Have Been Clarified: A Progress Report". Journal of the Physical Society of Japan. 78 (6): 062001. arXiv:0906.2045
 . Bibcode:2009JPSJ...78f2001I. doi:10.1143/JPSJ.78.062001.
. Bibcode:2009JPSJ...78f2001I. doi:10.1143/JPSJ.78.062001. - ↑ Prakash, J.; Singh, S. J.; Samal, S. L.; Patnaik, S.; Ganguli, A. K. (2008). "Potassium fluoride doped LaOFeAs multi-band superconductor: Evidence of extremely high upper critical field". EPL (Europhysics Letters). 84 (5): 57003. Bibcode:2008EL.....8457003P. doi:10.1209/0295-5075/84/57003.
- 1 2 Chen, X. H.; Wu, T.; Wu, G.; Liu, R. H.; Chen, H.; Fang, D. F. (2008). "Superconductivity at 43 K in SmFeAsO1–xFx". Nature. 453 (7196): 761–762. Bibcode:2008Natur.453..761C. doi:10.1038/nature07045. PMID 18500328.
- ↑ Shirage, Parasharam M.; Miyazawa, Kiichi; Kito, Hijiri; Eisaki, Hiroshi; Iyo, Akira (2008). "Superconductivity at 43 K at ambient pressure in the iron-based layered compound La1‑xYxFeAsOy". Physical Review B. 78 (17): 172503. Bibcode:2008PhRvB..78q2503S. doi:10.1103/PhysRevB.78.172503.
- ↑ Ren, Z. A.; Yang, J.; Lu, W.; Yi, W.; Che, G. C.; Dong, X. L.; Sun, L. L.; Zhao, Z. X. (2008). "Superconductivity at 52 K in iron based F doped layered quaternary compound Pr[O1–xFx]FeAs". Materials Research Innovations. 12 (3): 105. doi:10.1179/143307508X333686.
- ↑ Shirage, Parasharam M.; Miyazawa, Kiichi; Kihou, Kunihiro; Lee, Chul-Ho; Kito, Hijiri; Tokiwa, Kazuyasu; Tanaka, Yasumoto; Eisaki, Hiroshi; Iyo, Akira (2010). "Synthesis of ErFeAsO-based superconductors by the hydrogen doping method". EPL (Europhysics Letters). 92 (5): 57011. arXiv:1011.5022
 . Bibcode:2010EL.....9257011S. doi:10.1209/0295-5075/92/57011.
. Bibcode:2010EL.....9257011S. doi:10.1209/0295-5075/92/57011. - 1 2 Shirage, Parasharam M.; Kihou, Kunihiro; Lee, Chul-Ho; Kito, Hijiri; Eisaki, Hiroshi; Iyo, Akira (2011). "Emergence of Superconductivity in "32522" Structure of (Ca3Al2O5–y)(Fe2Pn2) (Pn = As and P)". Journal of the American Chemical Society. 133 (25): 9630–3. doi:10.1021/ja110729m. PMID 21627302.
- 1 2 Shirage, Parasharam M.; Kihou, Kunihiro; Lee, Chul-Ho; Kito, Hijiri; Eisaki, Hiroshi; Iyo, Akira (2010). "Superconductivity at 28.3 and 17.1 K in (Ca4Al2O6−y)(Fe2Pn2) (Pn=As and P)". Applied Physics Letters. 97 (17): 172506. arXiv:1008.2586
 . Bibcode:2010ApPhL..97q2506S. doi:10.1063/1.3508957.
. Bibcode:2010ApPhL..97q2506S. doi:10.1063/1.3508957. - ↑ Yang, Jie; Li, Zheng-Cai; Lu, Wei; Yi, Wei; Shen, Xiao-Li; Ren, Zhi-An; Che, Guang-Can; Dong, Xiao-Li; Sun, Li-Ling; Zhou, Fang; Zhao, Zhong-Xian (2008). "Superconductivity at 53.5 K in GdFeAsO1−δ". Superconductor Science and Technology. 21 (8): 082001. Bibcode:2008SuScT..21h2001Y. doi:10.1088/0953-2048/21/8/082001.
- 1 2 Yin, Yi; Zech, M.; Williams, T. L.; Wang, X. F.; Wu, G.; Chen, X. H.; Hoffman, J. E. (2009). "Scanning Tunneling Spectroscopy and Vortex Imaging in the Iron Pnictide Superconductor BaFe1.8Co0.2As2". Physical Review Letters. 102 (9): 97002. arXiv:0810.1048v2
 . Bibcode:2009PhRvL.102i7002Y. doi:10.1103/PhysRevLett.102.097002.
. Bibcode:2009PhRvL.102i7002Y. doi:10.1103/PhysRevLett.102.097002. - 1 2 Ren, Zhi-An; Che, Guang-Can; Dong, Xiao-Li; Yang, Jie; Lu, Wei; Yi, Wei; Shen, Xiao-Li; Li, Zheng-Cai; Sun, Li-Ling; Zhou, Fang; Zhao, Zhong-Xian (2008). "Superconductivity and phase diagram in iron-based arsenic-oxides ReFeAsO1−δ (Re = rare-earth metal) without fluorine doping". EPL (Europhysics Letters). 83: 17002. arXiv:0804.2582
 . Bibcode:2008EL.....8317002R. doi:10.1209/0295-5075/83/17002.
. Bibcode:2008EL.....8317002R. doi:10.1209/0295-5075/83/17002. - 1 2 Rotter, Marianne; Tegel, Marcus; Johrendt, Dirk (2008). "Superconductivity at 38 K in the Iron Arsenide (Ba1–xKx)Fe2As2". Physical Review Letters. 101 (10): 107006. arXiv:0805.4630
 . Bibcode:2008PhRvL.101j7006R. doi:10.1103/PhysRevLett.101.107006. PMID 18851249.
. Bibcode:2008PhRvL.101j7006R. doi:10.1103/PhysRevLett.101.107006. PMID 18851249. - 1 2 Shirage, Parasharam Maruti; Miyazawa, Kiichi; Kito, Hijiri; Eisaki, Hiroshi; Iyo, Akira (2008). "Superconductivity at 26 K in (Ca1–xNax)Fe2As2". Applied Physics Express. 1: 081702. Bibcode:2008APExp...1h1702M. doi:10.1143/APEX.1.081702.
- ↑ Satoru Matsuishi; Yasunori Inoue; Takatoshi Nomura; Hiroshi Yanagi; Masahiro Hirano; Hideo Hosono (2008). "Superconductivity Induced by Co-Doping in Quaternary Fluoroarsenide CaFeAsF". J. Am. Chem. Soc. 2008 (44): 14428–14429. doi:10.1021/ja806357j. PMID 18842039.
- ↑ Wu, G; Xie, Y L; Chen, H; Zhong, M; Liu, R H; Shi, B C; Li, Q J; Wang, X F; Wu, T; Yan, Y J; Ying, J J; Chen, X H (2009). "Superconductivity at 56 K in samarium-doped SrFeAsF". Journal of Physics: Condensed Matter. 21 (14): 142203. arXiv:0811.0761
 . Bibcode:2009JPCM...21n2203W. doi:10.1088/0953-8984/21/14/142203.
. Bibcode:2009JPCM...21n2203W. doi:10.1088/0953-8984/21/14/142203. - 1 2 Wang, X.C.; Liu, Q.Q.; Lv, Y.X.; Gao, W.B.; Yang, L.X.; Yu, R.C.; Li, F.Y.; Jin, C.Q. (2008). "The superconductivity at 18 K in LiFeAs system". Solid State Communications. 148 (11–12): 538–540. arXiv:0806.4688
 . Bibcode:2008SSCom.148..538W. doi:10.1016/j.ssc.2008.09.057.
. Bibcode:2008SSCom.148..538W. doi:10.1016/j.ssc.2008.09.057. - 1 2 Pitcher, Michael J.; Parker, Dinah R.; Adamson, Paul; Herkelrath, Sebastian J. C.; Boothroyd, Andrew T.; Ibberson, Richard M.; Brunelli, Michela; Clarke, Simon J. (2008). "Structure and superconductivity of LiFeAs". Chemical Communications (45): 5918–20. doi:10.1039/b813153h. PMID 19030538.
- 1 2 Tapp, Joshua H.; Tang, Zhongjia; Lv, Bing; Sasmal, Kalyan; Lorenz, Bernd; Chu, Paul C. W.; Guloy, Arnold M. (2008). "LiFeAs: An intrinsic FeAs-based superconductor with Tc=18 K". Physical Review B. 78 (6): 060505. arXiv:0807.2274
 . Bibcode:2008PhRvB..78f0505T. doi:10.1103/PhysRevB.78.060505.
. Bibcode:2008PhRvB..78f0505T. doi:10.1103/PhysRevB.78.060505. - 1 2 Chu, C.W.; Chen, F.; Gooch, M.; Guloy, A.M.; Lorenz, B.; Lv, B.; Sasmal, K.; Tang, Z.J.; Tapp, J.H.; Xue, Y.Y. (2009). "The synthesis and characterization of LiFeAs and NaFeAs". Physica C: Superconductivity. 469 (9–12): 326–331. arXiv:0902.0806
 . Bibcode:2009PhyC..469..326C. doi:10.1016/j.physc.2009.03.016.
. Bibcode:2009PhyC..469..326C. doi:10.1016/j.physc.2009.03.016. - 1 2 Parker, Dinah R.; Pitcher, Michael J.; Clarke, Simon J. (2008). "Structure and superconductivity of the layered iron arsenide NaFeAs". Chemical Communications. 2189 (16): 2189. arXiv:0810.3214
 . doi:10.1039/B818911K.
. doi:10.1039/B818911K. - ↑ Fong-Chi Hsu, et al. (2008). "Superconductivity in the PbO-type structure α-FeSe". PNAS. 105 (38): 14262–14264. Bibcode:2008PNAS..10514262H. doi:10.1073/pnas.0807325105. PMC 2531064
 . PMID 18776050.
. PMID 18776050. - ↑ Mizuguchi, Yoshikazu; Tomioka, Fumiaki; Tsuda, Shunsuke; Yamaguchi, Takahide; Takano, Yoshihiko (2008). "Superconductivity at 27 K in tetragonal FeSe under high pressure". Appl. Phys. Lett. 93 (15): 152505. arXiv:0807.4315
 . Bibcode:2008ApPhL..93o2505M. doi:10.1063/1.3000616.
. Bibcode:2008ApPhL..93o2505M. doi:10.1063/1.3000616. - ↑ Sasmal, K.; Lv, Bing; Lorenz, Bernd; Guloy, Arnold M.; Chen, Feng; Xue, Yu-Yi; Chu, Ching-Wu (2008). "Superconducting Fe-Based Compounds (A1–xSrx) Fe2As2 with A=K and Cs with Transition Temperatures up to 37 K". Physical Review Letters. 101 (10): 107007. Bibcode:2008PhRvL.101j7007S. doi:10.1103/physrevlett.101.107007. PMID 18851250.
- ↑ Zhang, S. J.; Wang, X. C.; Liu, Q. Q.; Lv, Y. X.; Yu, X. H.; Lin, Z. J.; Zhao, Y. S.; Wang, L.; Ding, Y.; Mao, H. K.; Jin, C. Q. (2009). "Superconductivity at 31 K in the "111"-type iron arsenide superconductor Na1−xFeAs induced by pressure". EPL (Europhysics Letters). 88 (4): 47008. Bibcode:2009EL.....8847008Z. doi:10.1209/0295-5075/88/47008.
- ↑ Deng, Z.; Wang, X. C.; Liu, Q. Q.; Zhang, S. J.; Lv, Y. X.; Zhu, J. L.; Yu, R. C.; Jin, C. Q. (2009). "A new "111" type iron pnictide superconductor LiFeP". EPL (Europhysics Letters). 87 (3): 37004. Bibcode:2009EL.....8737004D. doi:10.1209/0295-5075/87/37004.
- ↑ Day, C. (2009). "Iron-based superconductors". Physics Today. 62 (8): 36. Bibcode:2009PhT....62h..36D. doi:10.1063/1.3206093.
- ↑ Stewart, G. R. (2011). "Superconductivity in iron compounds". Rev. Mod. Phys. 83 (4): 1589. Bibcode:2011RvMP...83.1589S. doi:10.1103/revmodphys.83.1589.
- ↑ Yates, K A; Usman, I T M; Morrison, K; Moore, J D; Gilbertson, A M; Caplin, A D; Cohen, L F; Ogino, H; Shimoyama, J (2010). "Evidence for nodal superconductivity in Sr2ScFePO3". Superconductor Science and Technology. 23 (2): 022001. arXiv:0908.2902
 . Bibcode:2010SuScT..23b2001Y. doi:10.1088/0953-2048/23/2/022001.
. Bibcode:2010SuScT..23b2001Y. doi:10.1088/0953-2048/23/2/022001. - ↑ Dai, Jianhui; Si, Qimiao; Zhu, Jian-Xin; Abrahams, Elihu (2009-03-17). "Iron pnictides as a new setting for quantum criticality". Proceedings of the National Academy of Sciences. 106 (11): 4118–4121. doi:10.1073/pnas.0900886106. ISSN 0027-8424. PMC 2657431
 . PMID 19273850.
. PMID 19273850. - ↑ "Japanese scientists use alcoholic drinks to induce superconductivity". Phys.org. 2014-05-07.
- ↑ Suzuki, Miwa (2014-05-15). "Red wine offers clue to superconductive future". Phys.org.
- ↑ Deguchi, K; Mizuguchi, Y; Kawasaki, Y; Ozaki, T; Tsuda, S; Yamaguchi, T; Takano, Y (2011). "Alcoholic beverages induce superconductivity in FeTe1−xSx". Superconductor Science and Technology. 24 (5): 055008. arXiv:1008.0666v2
 . Bibcode:2011SuScT..24e5008D. doi:10.1088/0953-2048/24/5/055008.
. Bibcode:2011SuScT..24e5008D. doi:10.1088/0953-2048/24/5/055008. - ↑ Deguchi, K; Sato, D; Sugimoto, M; Hara, H; Kawasaki, Y; Demura, S; Watanabe, T; Denholme, S J; Okazaki, H; Ozaki, T; Yamaguchi, T; Takeya, H; Soga, T; Tomita, M; Takano, Y (2012). "Clarification as to why alcoholic beverages have the ability to induce superconductivity in Fe1+dTe1−xSx". Superconductor Science and Technology. 25 (8): 084025. arXiv:1204.0190v1
 . Bibcode:2012SuScT..25h4025D. doi:10.1088/0953-2048/25/8/084025.
. Bibcode:2012SuScT..25h4025D. doi:10.1088/0953-2048/25/8/084025.