Iron oxide

Iron oxides are chemical compounds composed of iron and oxygen. All together, there are sixteen known iron oxides and oxyhydroxides.[1]
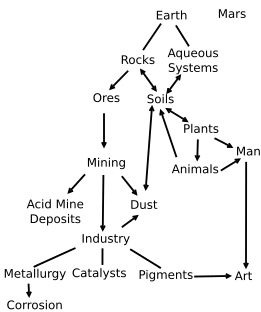
Iron oxides and oxide-hydroxides are widespread in nature, play an important role in many geological and biological processes, and are widely used by humans, e.g., as iron ores, pigments, catalysts, in thermite (see the diagram) and hemoglobin. Common rust is a form of iron(III) oxide. Iron oxides are widely used as inexpensive, durable pigments in paints, coatings and colored concretes. Colors commonly available are in the "earthy" end of the yellow/orange/red/brown/black range. When used as a food coloring, it has E number E172.
Oxides
- iron(II) oxide, wüstite (FeO)
- iron(II,III) oxides:
- iron(III) oxide (Fe2O3)
- alpha phase, hematite (α-Fe2O3)
- beta phase, (β-Fe2O3)
- gamma phase, maghemite (γ-Fe2O3)
- epsilon phase, (ε-Fe2O3)
Hydroxides
- iron(II) hydroxide (Fe(OH)2)
- iron(III) hydroxide (Fe(OH)3), (bernalite)
Oxide/hydroxides
- goethite (α-FeOOH),
- akaganéite (β-FeOOH),
- lepidocrocite (γ-FeOOH),
- feroxyhyte (δ-FeOOH),
- ferrihydrite ( approx.), or , better recast as
- high-pressure FeOOH
- schwertmannite (ideally or )[6]
- green rust ( where A− is Cl− or 0.5SO42−)
Microbial degradation
Several species of bacteria, including Shewanella oneidensis, Geobacter sulfurreducens and Geobacter metallireducens metabolically utilize solid iron oxides as a terminal electron acceptor, reducing Fe(III) oxides to Fe(II) containing oxides.[7]
See also
References
- ↑ Cornell, RM; Schwertmann, U (2003). The iron oxides: structure, properties, reactions, occurrences and uses. Wiley VCH. ISBN 3-527-30274-3.
- ↑ "Discovery of the recoverable high-pressure iron oxide Fe4O5". Oct 2011.
- ↑ "Synthesis of Fe5O6".
- 1 2 "Structural complexity of simple Fe2O3 at high pressures and temperatures".
- ↑ "The crystal structures of Mg2Fe2C4O13, with tetrahedrally coordinated carbon, and Fe13O19, synthesized at deep mantle conditions".
- ↑ http://www.mindat.org/min-7281.html Mindat
- ↑ Bretschger, O.; Obraztsova, A.; Sturm, C. A.; Chang, I. S.; Gorby, Y. A.; Reed, S. B.; Culley, D. E.; Reardon, C. L.; Barua, S.; Romine, M. F.; Zhou, J.; Beliaev, A. S.; Bouhenni, R.; Saffarini, D.; Mansfeld, F.; Kim, B.-H.; Fredrickson, J. K.; Nealson, K. H. (20 July 2007). "Current Production and Metal Oxide Reduction by Shewanella oneidensis MR-1 Wild Type and Mutants". Applied and Environmental Microbiology. 73 (21): 7003–7012. doi:10.1128/AEM.01087-07.
External links
| Wikimedia Commons has media related to Iron oxides. |
- Information from Nano-Oxides, Inc. on Fe2O3.
- http://chemed.chem.purdue.edu/demos/demosheets/12.3.html
- http://minerals.usgs.gov/minerals/pubs/commodity/iron_oxide/
- CDC - NIOSH Pocket Guide to Chemical Hazards
