Tetrakis(3,5-bis(trifluoromethyl)phenyl)borate
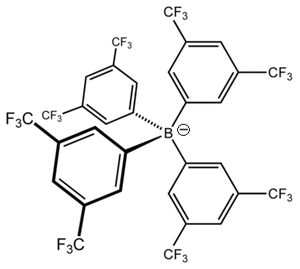
Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate is an anion with chemical formula [{3,5-(CF3)2C6H3}4B]−, which is commonly abbreviated as [BArF4]− as the boron atom (B) is surrounded by four fluorinated aryl (ArF) groups. It is sometimes referred to as Kobayashi's anion in honour of Hiroshi Kobayashi who led the team that first synthesised it,[1] but more commonly it is affectionately nicknamed "BARF."[2] BARF has a tetrahedral geometry around the central boron atom but each of the four surrounding aryl groups is aromatic and planar. The motivation for its preparation was the search for an anion which coordinates more weakly than the then-available ions hexafluorophosphate, tetrafluoroborate, or perchlorate.[3] Salts of this anion are known as solids and in both aqueous and non-aqueous solutions. BARF can be used in catalytic systems where the active site requires an anion which will not coordinate to the metal centre and interfere with the catalytic cycle, such as in the preparation of polyketones.[4]
Synthesis of sodium BARF
The sodium salt of the BARF ion is abbreviated NaBArF4 (or sometimes as NaBArF24[2] recognising the 24 fluorine atoms in each BARF ion). It was first prepared by a group led by Kobayashi and reported in 1984.[1] A Grignard reagent was prepared by reacting 1-iodo-3,5-bis(trifluoromethyl)benzene with magnesium metal in dry diethyl ether (Et2O) to form 3,5-bis(trifluoromethyl)phenylmagnesium iodide, ArFMgI where ArF = 3,5-(CF3)2C6H3. An ethereal solution of boron trifluoride is then added to this Grignard and the purified salt produced in 84% yield after workup and column chromatography.[1]
- BF3.OEt2 + 4 ArFMgI + NaF → 4 MgIF + NaBArF4 + OEt2
A safer synthesis has since been developed, utilising the magnesium-bromine exchange reaction between 1-bromo-3,5-bis(trifluoromethyl)benzene and isopropylmagnesium chloride to generate the required aryl-Grignard reagant, which is then reacted with sodium tetrafluoroborate.[2]
- NaBF4 + 4 ArFMgBr → NaBArF4 + 4 MgBrF
Properties
Non-coordinating anions are anions that interact only weakly with cations, a useful property when studying highly electrophilic cations.[5] In coordination chemistry, the term can also be used to refer to anions which are unlikely to bind directly to the metal centre of a complex. Hexafluorophosphate is a non-coordinating anion in both senses of the term.[6][7] Three widely used non-coordinating anions are hexafluorophosphate, tetrafluoroborate BF−
4, and perchlorate ClO−
4; of these, the hexafluorophosphate ion has the least coordinating ability[8] and it is deliberately used for this property. BARF was developed as a new non-coordinating anion in the 1990s, and is far less coordinating than even the hexafluorophosphate anion.[3]
Known compounds
Brookhart's acid is the salt of the BARF anion with the diethyl ether oxonium cation, [(Et2O)2H]BArF4. It can be formed from the sodium salt in diethyl ether in the presence of hydrogen chloride as sodium chloride is insoluble in diethyl ether, facilitating cation exchange.[3]
- NaBArF4 + HCl(g) + 2 Et2O → [(Et2O)2H]BArF4 + NaCl(s)
Synthesis of BARF salts with hexa(acetonitrile)metal(II) cations, [M(CH3CN)6]2+, are known for vanadium, chromium, manganese, iron, cobalt, and nickel.[9]
Application
Polyketones are a family of thermoplastic polymers formed by the copolymerisation of carbon monoxide and one or more alkenes (typically ethylene with propylene).[10][11] The process utilises a palladium(II) catalyst with a bidentate ligand like 2,2'-bipyridine or 1,10-phenanthroline (phen) with a non-coordinating BARF counterion, such as [(phen)Pd(CH3)(CO)]BArF4.[4] The preparation of the catalyst involves the reaction of a dimethyl palladium complex with Brookhart's acid in acetonitrile with loss of methane and the catalytic species is formed by uptake of carbon monoxide to displace acetonitrile.[4]
- [(Et2O)2H]BArF4 + [(phen)Pd(CH3)2] + MeCN → [(phen)Pd(CH3)(MeCN)]BArF4 + 2 Et2O + CH4
- [(phen)Pd(CH3)(MeCN)]BArF4 + CO → [(phen)Pd(CH3)(CO)]BArF4 + MeCN
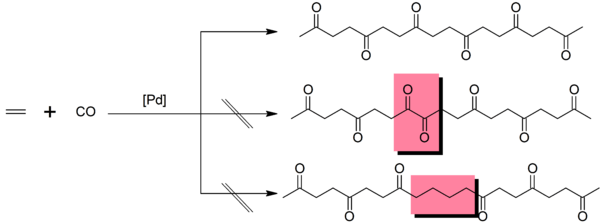
The mechanism involves migratory insertion[12] whereby the polymer chain is bound to the catalytic centre and grows by the sequential insertion of carbon monoxide and the alkene between the palladium atom and the existing chain. Defects occur when insertions do not alternate – that is, a carbon monoxide insertion follows a carbon monoxide insertion or an alkene insertion follows an alkene insertion – these are highlighted in red in the figure below. This catalyst produces a very low rate of defects due to the difference in Gibbs energy of activation of each insertion – the energy barrier to inserting an alkene immediately following an alkene insertion is ~12 kJ mol−1 higher than barrier to carbon monoxide insertion.[13] Use of monodentate phosphine ligands also leads to undesirable side-products[14] but bidentate phosphine ligands like 1,3-bis(diphenylphosphino)propane have been used industrially.[12]
References
- 1 2 3 Nishida, H.; Takada, N.; Yoshimura, M.; Sonods, T.; Kobayshi, H. (1984). "Tetrakis(3,5-bis(trifluoromethyl)phenyl)borate. Highly Lipophilic Stable Anionic Agent for Solvent-Extraction of Cations". Bull. Chem. Soc. Jap. 57 (9): 2600–2604. doi:10.1246/bcsj.57.2600.
- 1 2 3 Yakelis, N. A.; Bergman, R. G. (2005). "Safe Preparation and Purification of Sodium Tetrakis[(3,5-trifluoromethyl)phenyl]borate (NaBArF24): Reliable and Sensitive Analysis of Water in Solutions of Fluorinated Tetraarylborates". Organometallics. 24 (14): 3579–3581. doi:10.1021/om0501428.
- 1 2 3 Brookhart, M.; Grant, B.; Volpe, A. F. (1992). "[(3,5-(CF3)2C6H3)4B]−[H(OEt2)2]+: A Convenient Reagent for Generation and Stabilization of Cationic, Highly Electrophilic Organometallic Complexes". Organometallics. 11: 3920–3922. doi:10.1021/om00059a071.
- 1 2 3 Brookhart, M.; Rix, F. C.; DeSimone, J. M.; Barborak, J. C. (1992). "Palladium(II) catalysts for living alternating copolymerization of olefins and carbon monoxide". J. Am. Chem. Soc. 114 (14): 5894–5895. doi:10.1021/ja00040a082.
- ↑ Krossing, I.; Raabe, I. (2004). "Noncoordinating Anions - Fact or Fiction? A Survey of Likely Candidates". Angew. Chem. Int. Ed. 43 (16): 2066–2090. doi:10.1002/anie.200300620.
- ↑ Davies, J. A. (1996). Synthetic Coordination Chemistry: Principles and Practice. World Scientific. p. 165. ISBN 9810220847.
- ↑ Constant, S.; Lacour, J. (2005). Majoral, J.-P., ed. New Trends in Hexacoordinated Phosphorus Chemistry. New Aspects in Phosphorus Chemistry. 5. Springer. p. 3. ISBN 354022498X.
- ↑ Mayfield, H. G.; Bull, W. E. (1971). "Co-ordinating Tendencies of the Hexafluorophosphate Ion". J. Chem. Soc. A (14): 2279–2281. doi:10.1039/J19710002279.
- ↑ Buschman, W. E.; Miller, J. S. (2002). "Synthesis of [MII(NCMe)6]2+ (M = V, Cr, Mn, Fe, Co, Ni) salts of tetra[3,5-bis(trifluoromethyl)phenyl]borate". Inorg. Synth. 33: 83. doi:10.1002/0471224502.ch2.
- ↑ Drent, E.; Mul, W. P.; Smaardijk, A. A. (2001). "Polyketones". Encyclopedia Of Polymer Science and Technology. doi:10.1002/0471440264.pst273.
- ↑ Bianchini, C.; Meli, A. (2002). "Alternating copolymerization of carbon monoxide and olefins by single-site metal catalysis". Coord. Chem. Rev. 225 (1-2): 35–66. doi:10.1016/S0010-8545(01)00405-2.
- 1 2 Shultz, C. S.; Ledford, J.; Desimone, J. M.; Brookhart, M. (2000). "Kinetic studies of migratory insertion reactions at the (1,3-bis(diphenylphosphino)propane)Pd(II) center and their relationship to the alternating copolymerization of ethylene and carbon monoxide". J. Am. Chem. Soc. 122 (27): 6351–6356. doi:10.1021/ja994251n.
- ↑ Rix, F. C.; Brookhart, M.; White, P. S. (1996). "Mechanistic studies of the palladium(II)-catalyzed copolymerization of ethylene with carbon monoxide". J. Am. Chem. Soc. 118 (20): 4746–4764. doi:10.1021/ja953276t.
- ↑ Drent, E.; Budzelaar, P. H. M. (1996). "Palladium-catalyzed alternating copolymerization of alkenes and carbon monoxide". Chem. Rev. 96 (2): 663–682. doi:10.1021/cr940282j.