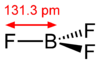Boron trifluoride
| |||
| Names | |||
|---|---|---|---|
| Other names
Boron fluoride, Trifluoroborane | |||
| Identifiers | |||
| 7637-07-2 13319-75-0 (dihydrate) | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:33093 | ||
| ChemSpider | 6116 | ||
| ECHA InfoCard | 100.028.699 | ||
| EC Number | 231-569-5 | ||
| PubChem | 6356 | ||
| RTECS number | ED2275000 | ||
| UNII | 7JGD48PX8P | ||
| UN number | Compressed: 1008. Boron trifluoride dihydrate: 2851. | ||
| |||
| |||
| Properties | |||
| BF3 | |||
| Molar mass | 67.82 g/mol (anhydrous) 103.837 g/mol (dihydrate) | ||
| Appearance | colorless gas (anhydrous) colorless liquid (dihydrate) | ||
| Density | 0.00276 g/cm3 (anhydrous gas) 1.64 g/cm3 (dihydrate) | ||
| Melting point | −126.8 °C (−196.2 °F; 146.3 K) | ||
| Boiling point | −100.3 °C (−148.5 °F; 172.8 K) | ||
| exothermic decomposition [1] (anhydrous) very soluble (dihydrate) | |||
| Solubility | soluble in benzene, toluene, hexane, chloroform and methylene chloride | ||
| Vapor pressure | >50 atm (20°C)[2] | ||
| 0 D | |||
| Thermochemistry | |||
| 50.46 J/mol K | |||
| Std molar entropy (S |
254.3 J/mol K | ||
| Std enthalpy of formation (ΔfH |
-1137 kJ/mol | ||
| Gibbs free energy (ΔfG˚) |
-1120 kJ/mol | ||
| Hazards[3][4] | |||
| Safety data sheet | ICSC | ||
| GHS pictograms |   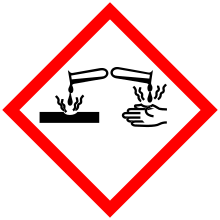 | ||
| GHS signal word | DANGER | ||
| H330, H314 [note 1] | |||
| EU classification (DSD) |
Very toxic (T+) Corrosive (C) | ||
| R-phrases | R14, R26, R35 | ||
| S-phrases | (S1/2), S9, S26, S28, S36/37/39, S45 | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) |
1227 ppm (mouse, 2 hr) 39 ppm (guinea pig, 4 hr) 418 ppm (rat, 4 hr)[5] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
C 1 ppm (3 mg/m3)[2] | ||
| REL (Recommended) |
C 1 ppm (3 mg/m3)[2] | ||
| IDLH (Immediate danger) |
25 ppm[2] | ||
| Related compounds | |||
| Other anions |
Boron trichloride Boron tribromide Boron triiodide | ||
| Other cations |
Aluminium fluoride Gallium(III) fluoride Indium(III) fluoride Thallium(III) fluoride | ||
| Related compounds |
Boron monofluoride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Boron trifluoride is the inorganic compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bonding
The geometry of a molecule of BF3 is trigonal planar. Its D3h symmetry conforms with the prediction of VSEPR theory. The molecule has no dipole moment by virtue of its high symmetry. The molecule is isoelectronic with the carbonate anion, CO32−.
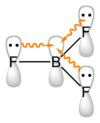
BF3 is commonly referred to as "electron deficient," a description that is reinforced by its exothermic reactivity toward Lewis bases.
In the boron trihalides, BX3, the length of the B-X bonds (1.30 Å) is shorter than would be expected for single bonds,[6] and this shortness may indicate stronger B-X π-bonding in the fluoride. A facile explanation invokes the symmetry-allowed overlap of a p orbital on the boron atom with the in-phase combination of the three similarly oriented p orbitals on fluorine atoms.[6] Others point to the ionic nature of the bonds in BF3.[7]
Synthesis and handling
BF3 is manufactured by the reaction of boron oxides with hydrogen fluoride:
- B2O3 + 6 HF → 2 BF3 + 3 H2O
Typically the HF is produced in situ from sulfuric acid and fluorite (CaF2).[8] Approximately 2300-4500 tonnes of boron trifluoride are produced every year.[9]
On a laboratory scale, BF3 is produced by the thermal decomposition of diazonium salts:[10]
Alternatively the chemical can be synthesized from Sodium tetrafluoroborate, Boron trioxide, and Sulfuric acid:[11]
- 6 NaBF4 + B2O3 + 6 H2SO4 → 8 BF3 + 6 NaHSO4 + 3 H2O
Anhydrous boron trifluoride has a boiling point of −100.3 C and a critical temperature of −12.3 C, so that it can be stored as a refrigerated liquid only between those temperatures. Storage or transport vessels should be designed to withstand internal pressure, since a refrigeration system failure could cause pressures to rise to the critical pressure of 49.85 bar (4.985 MPa).[12]
Boron trifluoride is corrosive. Suitable metals for equipment handling boron trifluoride include stainless steel, monel, and hastelloy. In presence of moisture it corrodes steel, including stainless steel. It reacts with polyamides. Polytetrafluoroethylene, polychlorotrifluoroethylene, polyvinylidene fluoride, and polypropylene show satisfactory resistance. The grease used in the equipment should be fluorocarbon based, as boron trifluoride reacts with the hydrocarbon-based ones.[13]
Reactions
Unlike the aluminium and gallium trihalides, the boron trihalides are all monomeric. They undergo rapid halide exchange reactions:
- BF3 + BCl3 → BF2Cl + BCl2F
Because of the facility of this exchange process, the mixed halides cannot be obtained in pure form.
Boron trifluoride is a versatile Lewis acid that forms adducts with such Lewis bases as fluoride and ethers:
Tetrafluoroborate salts are commonly employed as non-coordinating anions. The adduct with diethyl ether, boron trifluoride diethyl etherate or just boron trifluoride etherate (BF3 · O(Et)2) is a conveniently handled liquid and consequently is widely encountered as a laboratory source of BF3. It is stable as a solution in ether, but not stoichiometrically. Another common adduct is the adduct with dimethyl sulfide (BF3 · S(Me)2), which can be handled as a neat liquid.
Comparative Lewis acidity
All three lighter boron trihalides, BX3 (X = F, Cl, Br) form stable adducts with common Lewis bases. Their relative Lewis acidities can be evaluated in terms of the relative exothermicities of the adduct-forming reaction. Such measurements have revealed the following sequence for the Lewis acidity:
- BF3 < BCl3 < BBr3 (strongest Lewis acid)
This trend is commonly attributed to the degree of π-bonding in the planar boron trihalide that would be lost upon pyramidalization of the BX3 molecule.[14] which follows this trend:
- BF3 > BCl3 > BBr3 (most easily pyramidalized)
The criteria for evaluating the relative strength of π-bonding are not clear, however.[6] One suggestion is that the F atom is small compared to the larger Cl and Br atoms, and the lone pair electron in pz of F is readily and easily donated and overlapped to empty pz orbital of boron. As a result, the pi donation of F is greater than that of Cl or Br.
In an alternative explanation, the low Lewis acidity for BF3 is attributed to the relative weakness of the bond in the adducts F3B-L.[15][16]
Hydrolysis
Boron trifluoride reacts with water to give boric acid and fluoroboric acid. The reaction commences with the formation of the aquo adduct, H2O-BF3, which then loses HF that gives fluoboric acid with boron trifluoride.[17]
- 4 BF3 + 3 H2O → 3 HBF4 + "B(OH)3"
The heavier trihalides do not undergo analogous reactions, possibly due to the lower stability of the tetrahedral ions BX4− (X = Cl, Br). Because of the high acidity of fluoroboric acid, the fluoroborate ion can be used to isolate particularly electrophilic cations, such as diazonium ions, that are otherwise difficult to isolate as solids.
Uses
Organic chemistry
Boron trifluoride is most importantly used as a reagent in organic synthesis, typically as a Lewis acid.[9][18] Examples include:
- initiates polymerisation reactions of unsaturated compounds, such as polyethers
- as a catalyst in some isomerization, acylation,[19] alkylation, esterification, dehydration,[20] condensation, Mukaiyama aldol addition, and other reactions[21]
Niche uses
Other, less common uses for boron trifluoride include:
- applied as dopant in ion implantation
- p-type dopant for epitaxially grown silicon
- used in sensitive neutron detectors in ionization chambers and devices to monitor radiation levels in the Earth's atmosphere
- in fumigation
- as a flux for soldering magnesium
- to prepare diborane[11]
Discovery
Boron trifluoride was discovered in 1808 by Joseph Louis Gay-Lussac and Louis Jacques Thénard, who were trying to isolate "fluoric acid" (i.e., hydrofluoric acid) by combining calcium fluoride with vitrified boric acid. The resulting vapours failed to etch glass, so they named it fluoboric gas.[22][23]
Notes
- ↑ Within the European Union, the following additional hazard statement (EUH014) must also be displayed on labelling: Reacts violently with water.
References
- ↑ http://www.nap.edu/openbook.php?record_id=4911&page=266
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0062". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Index no. 005-001-00-X of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 341.
- ↑ "Boron trifluoride", Pocket Guide to Chemical Hazards, U.S. Department of Health and Human Services (NIOSH) Publication No. 2005-149, Washington, DC: Government Printing Office, 2005, ISBN 9780160727511.
- ↑ "Boron trifluoride". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Gillespie, Ronald J. (1998). "Covalent and Ionic Molecules: Why Are BeF2 and AlF3 High Melting Point Solids whereas BF3 and SiF4 Are Gases?". Journal of Chemical Education. 75 (7): 923. doi:10.1021/ed075p923.
- ↑ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- 1 2 Brotherton, R. J.; Weber, C. J.; Guibert, C. R.; Little, J. L. (2005), "Boron Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a04_309
- ↑ Flood, D. T. (1933). "Fluorobenzene". Org. Synth. 13: 46.; Coll. Vol., 2, p. 295
- 1 2 Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry Vol. 1, 2nd Ed. Newyork: Academic Press. p. 220 & 773. ISBN 978-0121266011.
- ↑ Yaws, C. L., ed. (1999). Chemical Properties Handbook. McGraw-Hill. p. 25.
- ↑ "Boron trifluoride". Gas Encyclopedia. Air Liquide.
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ↑ Boorman, P. M.; Potts, D. (1974). "Group V Chalcogenide Complexes of Boron Trihalides". Canadian Journal of Chemistry. 52 (11): 2016–2020. doi:10.1139/v74-291.
- ↑ Brinck, T.; Murray, J. S.; Politzer, P. (1993). "A Computational Analysis of the Bonding in Boron Trifluoride and Boron Trichloride and their Complexes with Ammonia". Inorganic Chemistry. 32 (12): 2622–2625. doi:10.1021/ic00064a008.
- ↑ Wamser, C. A. (1951). "Equilibria in the System Boron Trifluoride–Water at 25°". Journal of the American Chemical Society. 73 (1): 409–416. doi:10.1021/ja01145a134.
- ↑ Heaney, H. (2001). "Boron Trifluoride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rb250. ISBN 0-471-93623-5.
- ↑ Mani, Rama I.; Erbert, Larry H.; Manise, Daniel (1991). "Boron Trifluoride in the Synthesis of Plant Phenolics: Synthesis of Phenolic Ketones and Phenyl Stryl Ketones" (PDF). Journal of Tennessee Academy of Science. 66 (1): 1–8. Retrieved 27 October 2016.
- ↑ Sowa, F. J.; Hennion, G. F.; Nieuwland, J. A. (1935). "Organic Reactions with Boron Fluoride. IX. The Alkylation of Phenol with Alcohols". Journal of the American Chemical Society. 57 (4): 709–711. doi:10.1021/ja01307a034.
- ↑ "Boron Trifluoride (BF3) Applications". Honeywell.
- ↑ Gay-Lussac, J. L.; Thénard, L. J. (1809). "Sur l'acide fluorique". Annales de Chimie. 69: 204–220.
- ↑ Gay-Lussac, J. L.; Thénard, L. J. (1809). "Des propriétés de l'acide fluorique et sur-tout de son action sur le métal de la potasse". Mémoires de Physique et de Chimie de la Société d’Arcueil. 2: 317–331.
External links
- "Safety and Health Topics: Boron Trifluoride". OSHA.
- "BORON TRIFLUORIDE ICSC: 0231". International Chemical Safety Cards. CDC.
- "Boron & Compounds: Overview". National Pollutant Inventory. Australian Government.
- "Fluoride Compounds: Overview". National Pollutant Inventory. Australian Government.
- "Boron trifluoride". WebBook. NIST.
- "Boron Trifluoride (BF3) Applications". Honeywell.