Linaclotide
 | |
| Clinical data | |
|---|---|
| Trade names | Linzess |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | A06AX04 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
851199-59-2 |
| PubChem (CID) | 16158208 |
| IUPHAR/BPS | 5017 |
| ChemSpider |
17314504 |
| UNII |
N0TXR0XR5X |
| KEGG |
D09355 |
| Chemical and physical data | |
| Formula | C59H79N15O21S6 |
| Molar mass | 1526.74 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Linaclotide (marketed under the trade name Linzess in the US[1] and Mexico, and as Constella in Canada[2] and many other countries[3]) is a peptide agonist of guanylate cyclase 2C. It was approved by the FDA in August 2012 and by the European Medicines Agency (EMA)[4] for the treatment in adults of constipation-predominant irritable bowel syndrome (IBS-C) and chronic idiopathic constipation (CIC).[5]
Background
The National Institutes of Health (NIH) estimate that as many as 20% of Americans may experience irritable bowel syndrome, with approximately one-third of those affected experiencing constipation often accompanied by abdominal pain, affecting as many as 10 million Americans. Laxatives can assist with constipation but do not treat pain, while use of opiates to treat pain can aggravate constipation. While low-cost laxatives and pain killers would likely be tried first, linaclotide targets both associated conditions in a once-daily pill.[6]
Linaclotide is a peptide agonist mimic of endogenous guanylin and uroguanylin, both of which activate the cell surface receptor of guanylate cyclase 2C (GC-C).[7]:108–109[8][9] The medication binds to the surface of the intestinal epithelial cells.[8] Linaclotide is minimally absorbed and it is undetectable in the systemic circulation at therapeutic doses.[7]:108 Activation of GC-C increases cyclic guanosine monophosphate (cGMP).[8] Elevated cGMP stimulates secretion of chloride and bicarbonate and water into the intestinal lumen, mainly by way of cystic fibrosis transmembrane conductance regulator (CFTR) ion channel activation.[8][10] This results in increased intestinal fluid and accelerated transit.[8] By elevating cGMP, linaclotide is also considered to reduce activation of colonic sensory neurons, reducing pain;[8][11] and activates colonic motor neurons, which increases smooth muscle contraction and thus promotes bowel movements.
Clinical trials
The FDA approval of linaclotide was based on four large randomized trials, two for each indication: IBS-C and CIC.[8] The approved dose for constipation-predominant irritable bowel syndrome (IBS-C) is 290 micrograms daily.[5][9] The approved dose for chronic idiopathic constipation CIC is 145 mcg daily; no benefit was seen in CIC by using 290 micrograms instead of 145 mcg daily.[8] In both situations, the most common side effect was diarrhea.[8]
In IBS-C, the phase III randomized double-blind clinical trials both showed some improvement in pain and constipation with the medication in comparison to placebo.[8]:11-13 Pain reduction started in the first week on the medication, with further improvement seen by weeks six to nine.[8]
In CIC, some effect on bowel habits was seen during the first week of treatment, reaching its maximum within that interval. Similarly, the effect returned toward baseline within one week of discontinuing the medication.[8]:13-14[11]:figure 2B An open-label safety study in CIC using linaclotide for up to a year found diarrhea to be the most frequently reported adverse event.[11]
Distribution and licensing
Under a partnership agreement announced in 2007 between Forest Laboratories and Microbia (as Ironwood Pharmaceuticals was then known), Forest would pay $70 million in licensing fees towards the development of linaclotide, with profits shared between the two companies.[12] Distribution rights in the United States will be shared with Forest Laboratories, with Almirall distributing linaclotide in Europe and Astellas Pharma in Asia.[6]
Chemistry
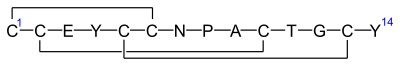
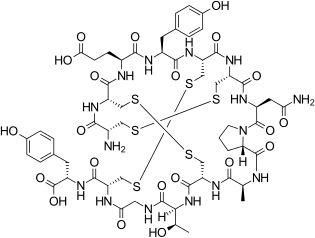
Linaclotide is a peptide consisting of 14 amino acids. Systemic absorption is minimal.[7]:108[11] The sequence is
H–Cys1–Cys2–Glu3–Tyr4–Cys5–Cys6–Asn7–Pro8–Ala9–Cys10–Thr11–Gly12–Cys13–Tyr14–OH
There are three disulfide bonds: between Cys1 and Cys6, between Cys2 and Cys10, and between Cys5 and Cys13.[13]
References
- ↑ http://www.fda.gov/downloads/Drugs/DrugSafety/UCM318437.pdf
- ↑ Constella (linaclotide) [product monograph]. Vaughan, Ontario, Canada: Forest Laboratories Canada Inc; May 2014.
- ↑ European Medications Agency ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002490/WC500135622.pdf
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4107700/
- 1 2 "FDA approves Linzess to treat certain cases of irritable bowel syndrome and constipation" Food and Drug Administration news release, August 30, 2012. Accessed April 28, 2016.
- 1 2 Pollack, Andrew. "Drug for Irritable Bowel Achieves Goals in Trial", The New York Times, September 13, 2010. Accessed September 14, 2010.
- 1 2 3 Hussain ZH, Everhart K, Lacy BE (2015). "Treatment of Chronic Constipation: Prescription Medications and Surgical Therapies". Gastroenterol Hepatol (NY). 11 (2): 104–14. PMC 4836568
 . PMID 27099579.
. PMID 27099579. - 1 2 3 4 5 6 7 8 9 10 11 12 Linzess package insert, Allergan, Plc, revised November 2015. Accessed August 18, 2016.
- 1 2 Love, Bryan L.; Johnson, Audrey; Smith, Lisa S. (2014-07-01). "Linaclotide: A novel agent for chronic constipation and irritable bowel syndrome". American Journal of Health-System Pharmacy. 71 (13): 1081–1091. doi:10.2146/ajhp130575. ISSN 1079-2082. PMID 24939497.
- ↑ Yu SW, Rao SS (2014). "Advances in the management of constipation-predominant irritable bowel syndrome: the role of linaclotide". Therap Adv Gastroenterol. 7 (5): 193–205. doi:10.1177/1756283X14537882. PMC 4107700
 . PMID 25177366.
. PMID 25177366. - 1 2 3 4 Corsetti M, Tack J (2013). "Linaclotide: A new drug for the treatment of chronic constipation and irritable bowel syndrome with constipation". United European Gastroenterol J. 1 (1): 7–20. doi:10.1177/2050640612474446. PMC 4040778
 . PMID 24917937.
. PMID 24917937. - ↑ Staff. "Daily International Pharma Alert", FDANews, September 17, 2007, Vol. 4 No. 182. Accessed September 15, 2010.
- ↑ Albericio, F; Giraud, M; Gongora, M; et al. "Solid-Phase Synthesis of the Cys-rich Peptide Linaclotide" (PDF).
External links
- Linzess information Drugs com Accessed October 23, 2013.