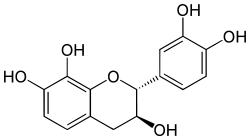
Mesquitol
 | |
| Names | |
|---|---|
| IUPAC name
3,4-Dihydro-2alpha- (3,4-dihydroxyphenyl) -2H-1-benzopyran-3beta,7,8-triol | |
| Other names
(-)-mesquitol | |
| Identifiers | |
| 109671-55-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 9208756 |
| PubChem | 11033582 |
| |
| |
| Properties | |
| C15H14O6 | |
| Molar mass | 290.26 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Mesquitol is a flavan-3-ol, a type of flavonoid.[1]
Prosopis juliflora, a mesquite found in Kenya, shows unusual amount of (-)-mesquitol from its heartwood.[2]
Mesquitol with its pyrogallol-type A-ring is more susceptible to quinone formation at this ring leading to aryl–aryl bond formation at carbon 5. The structural moieties constitute the proteracacinidin class of proanthocyanidins.[3] Mesquitol-(5→8)-catechin atropisomers can be isolated from Prosopis glandulosa.[4]
References
- ↑ Mesquitol on metabolomics.jp
- ↑ Unusual amount of (-)-mesquitol from the heartwood of Prosopis juliflora. Sirmah Peter, Dumarcay Stephane, Masson Eric and Gerardin Philippe, Natural Product Research, January 2009, Volume 23, Number 2, pages 183-189, doi:10.1080/14786410801940968
- ↑ Oligomeric proanthocyanidins: naturally occurring O-heterocycles. Daneel Ferreira and Desmond Slade, Nat. Prod. Rep., 2002, volume 19, pages 517–541, doi:10.1039/b008741f
- ↑ Synthesis of condensed tannins. Part 17. Oligomeric (2R,3S)-3,3′,4′,7,8-pentahydroxyflavans: atropisomerism and conformation of biphenyl and m-terphenyl analogues from Prosopis glandulosa(‘mesquite’). Esmé Young, Edward V. Brandt, Desmond A. Young, Daneel Ferreira and David G. Roux, J. Chem. Soc., Perkin Trans. 1, 1986, pages 1737-1749, doi:10.1039/P19860001737
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.