Acetylide
| | |
| Identifiers | |
|---|---|
| 29075-95-4 (HC≡C−) 34846-56-5 (C22−) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 11316391 (HC≡C−) 6113 (C22−) |
| |
| |
| Properties | |
| HC− 2 | |
| Molar mass | 25.03 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Acetylide is a carbanion with the chemical formula HC≡C−. The classification includes saline carbides and organometallic compounds of terminal alkynes and acetylene. Substituted acetylides, which have the general structure RC≡C− (where R is an organic side chain), are useful reagents in organic synthesis.
Structure
The double or single deprotonation product of acetylene is often referred to as an acetylide anion of the formula C2−
2 or HC−
2 respectively. The doubly deprotonated product has a closed shell ground state of 1Σg+, making it isoelectronic to a neutral molecule N2,[1] This chemical structure affords it some stability in the gas phase but makes it reactive to strong bases in the liquid phase (see acetylene bonding).
Although acetylides are often described as anions in salts, acetylides are more accurately described as complex structures of an anion strongly bonded to a metal cation. Nevertheless, the anion names are used for salt-like materials such as alkali metal acetylides, copper acetylide (Cu2C2), lithium hydrogenacetylide (LiC2H), and silver methylacetylide (AgCH3C2). Some metal acetylides are traditionally called carbides. For example, lithium carbide and calcium carbide are really derivatives of C2−
2.
Preparation
Terminal alkynes are weak acids and form acetylides by behaving as conjugate bases.[2] In the presence of an inorganic or organometallic base, the alkyne undergoes deprotonation to form a metal acetylide:
- RC≡CH + R"M ↔ R"H + RC≡CM
The formation of the acetylide depends upon several factors such as the pKa of the alkyne, the pKb of the conjugate acid, the valency of the metal, and solvent characteristics.
Alkali metal acetylides
Forming pure acetylides from alkali metals often requires the use of organometallic[3] or inorganic[4] superbases in solvents which are less acidic than the terminal alkyne (e.g. anhydrous solvents or liquid ammonia).
Lithium amide,[2] LiHMDS,[5] or organolithium reagents, such as butyllithium,[3] are frequently used form lithium acetylides:
Sodium or potassium acetylides can be prepared from various inorganic reagents (e.g. sodium amide)[4] or from their elemental metals, often at room temperature and atmospheric pressure.[2]
Copper and silver acetylides
Copper(I) acetylide can be prepared by passing acetylene through an aqueous solution of copper(I) chloride because of a low solubility equilibrium.[2] Similarly, silver acetylides can be obtained from silver nitrate.
Calcium and lithium carbides
Calcium carbide is prepared by heating carbon with lime CaO at approximately 2000 °C. A similar process is used to produce lithium carbide.
Reactions
Alkynations
Acetylide ions are very useful in organic chemistry reactions in combining carbon chains, particularly addition reactions. One type of reaction displayed by acetylides are nucleophilic addition reactions with ketones to form α-alkynyl alcohols.
For example, in the following reaction (scheme 1), the alkyne proton of ethyl propiolate is deprotonated by n-butyllithium at -78 °C to form lithium ethyl propiolate to which cyclopentanone is added forming a lithium alkoxide. Acetic acid is added to remove lithium and liberate the free alcohol.[6]

Modifications
Several modifications of alkynation reactions are known:
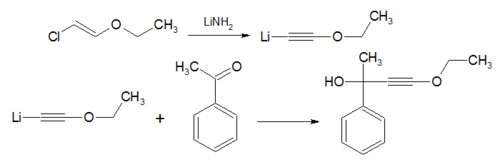
- In the Arens–van Dorp Synthesis the compound ethoxyacetylene[7] is converted to an Grignard reagent and reacted with a ketone, the reaction product is a propargyl alcohol.[8][9]
- The Isler modification is a modification of Arens–van Dorp Synthesis where ethoxyacetylene is replaced by β-chlorovinyl ether and lithium amide.[8]
- In the Favorskii reaction ketones and acetylenic compounds react in presence of alkali.[2]
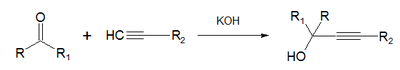
Coupling reactions
Acetylides are sometimes intermediates in coupling reactions. Examples include Sonogashira coupling, Cadiot-Chodkiewicz coupling, Glaser coupling and Eglinton coupling.
Hazards
Some acetylides are notoriously explosive. Formation of acetylides poses a risk in handling of gaseous acetylene in presence of metals such as mercury, silver or copper, or alloys with their high content (brass, bronze, silver solder).
See also
- Ethynyl
- Ethynyl radical
- Diatomic carbon - Neutral C2
- Acetylenediol
References
- ↑ Sommerfeld, T.; Riss, U.; Meyer, H.-D.; Cederbaum, L. (August 1997). "Metastable C22- Dianion". Physical Review Letters. 79 (7): 1237–1240. doi:10.1103/PhysRevLett.79.1237.
- 1 2 3 4 5 Viehe, Heinz Günter (1969). Chemistry of Acetylenes (1st ed.). New York: Marcel Dekker, inc. pp. 170–179 & 225–241. doi:10.1002/ange.19720840843.
- 1 2 Midland, M. M.; McLoughlin, J. I.; Werley, Ralph T. (Jr.) (1990). "Preparation and Use of Lithium Acetylide: 1-Methyl-2-ethynyl-endo-3,3-dimethyl-2-norbornanol". Organic Syntheses. 68: 14. doi:10.15227/orgsyn.068.0014.
- 1 2 Coffman, Donald D. (1940). "Dimethylethhynylcarbinol". Organic Syntheses. 40: 20. doi:10.15227/orgsyn.020.0040.
- ↑ Reich, Melanie (Aug 24, 2001). "Addition of a lithium acetylide to an aldehyde; 1-(2-pentyn-4-ol)-cyclopent-2-en-1-ol". ChemSpider Synthetic Pages. p. 137. doi:10.1039/SP137.
- ↑ Midland, M. Mark; Tramontano, Alfonso; Cable, John R. (1980). "Synthesis of alkyl 4-hydroxy-2-alkynoates". The Journal of Organic Chemistry. 45 (1): 28–29. doi:10.1021/jo01289a006.
- ↑ Jones, E. R. H.; Eglinton, Geoffrey; Whiting, M. C.; Shaw, B. L. (1954). "Ethoxyacetylene". Organic Syntheses. 34: 46. doi:10.15227/orgsyn.034.0046.
- 1 2 Wang, Zerong, ed. (2009). "Arens-van Dorp Reaction (Isler Modification)". Comprehensive Organic Name Reactions and Reagents (1st ed.). Hoboken, NJ: Wiley-Interscience. doi:10.1002/9780470638859.conrr023. ISBN 9780471704508.
- ↑ Van Dorp, D. A.; Arens, J. F. (1947). "Synthesis of Vitamin A Aldehyde-". Nature. 160 (4058): 189. doi:10.1038/160189a0.