Metal carbonyl cluster
In chemistry, a metal carbonyl cluster is a compound that contains two or more metals linked in part by metal-metal bonds and containing carbon monoxide (CO) as the exclusive or predominant ligand. Simple examples include Fe2(CO)9, Fe3(CO)12, Mn2(CO)10.[1] High nuclearity clusters include [Rh13(CO)24H3]2− and the stacked Pt3 triangules [Pt3n(CO)6n]2− (n = 2–6).[2]

Classes of carbonyl clusters
Binary metal carbonyl clusters
Binary carbonyl clusters consist only of metal and CO. They are the most widely studied and used metal carbonyl clusters. They arise in general by the condensation of unsaturated metal carbonyls. Dissociation of CO from Ru(CO)5 would give Ru(CO)4, which could trimerize to Ru3(CO)12. The reaction mechanisms are more complicated than this simple scenario. Condensation of low molecular weight metal carbonyls requires decarbonylation, which can be induced thermally, photochemically, or using various reagents. The nuclearity (number of metal centers) of binary metal carbonyl clusters is usually no greater than six.
| metal | parent carbonyl | cluster |
|---|---|---|
| Fe | Fe(CO)5 | Fe2(CO)9, Fe3(CO)12 |
| Ru | Ru(CO)5 | Ru3(CO)12 |
| Os | Os(CO)5 | Os3(CO)12 |
| Co | Co2(CO)8 | Co4(CO)12 |
| Rh | Rh2(CO)8 | Rh4(CO)12 |
| Ir | Ir2(CO)8 | Ir4(CO)12 |
"Chini Clusters"
Chini clusters follow the general formula of [Pt3(CO)6]n2−,1 < n < 10.[3] These clusters are prepared by reduction of hexachloroplatinate with strongly basic methanol under an atmosphere of CO.[4] These clusters consist of stacks of triangularly shaped Pt3 subunits. Although these clusters were first reported in 1969 by Chatt and Booth, their structure were not established until Chini and Longoni’s work in 1976.[3][4]
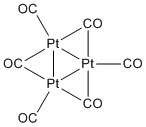
Chini clusters are based on a planar triangular building block that can be condensed multiple units forming chains usually anywhere from two to ten units long. The chains are formed by stacking of the planar units, extending through platinum to platinum bonds forming trigonal prismatic clusters. Within a triangular unit, the platinum-platinum bond lengths are 2.65 Å and between units the Pt---Pt bond lengths are 3.05 Å. Cluster structure is easily disrupted by deposition onto surfaces such as carbon or silicon, where the chains are broken, but the triangular subunits remain intact.[5] The tetramer [Pt3(CO)6]42− is the most common member of this series of clusters.[6] These clusters undergo reversible redox. They catalyze the hydrogenation of alkenes, ketones, aldehydes.
Chini clusters can also be converted heterometal clusters and catalyze pH driven redox reactions and transport. First, the Chini clusters are the source of platinum atoms for the mixed metal cluster synthesis.[3] For instance, the reaction [Pt12(CO)24]2− with [Ag(PPh3)4]+ produces heterometal cluster [Pt3Ag(CO)3(PPh3)5]+. Second, the Chini clusters with redox properties act as a catalyst that helps transport sodium ions and electrons in the same direction across a liquid membrane, driven by pH-gradient. The [Pt3(CO)6]n-12− platinum clusters, where n = 4 – 6, are reduced by HO-.
(n-1)[Pt3(CO)6]n2− + 2OH− ↔ n[Pt3(CO)6]n-12− + H2O + 1/2O2
Metal carbido clusters
Although the nuclearity of binary metal carbonyl clusters is usually six or fewer, carbido clusters often have higher nuclearities. Metal carbonyls of the iron and cobalt triads are well known to form carbido derivatives. Examples include [Rh6C(CO)15]2−[7] and [Ru6C(CO)16]2−.[8] Carbonyl carbides exist not only with fully encapsulated carbon (e.g., [Fe6C(CO)16]2−) but also with exposed carbon centres as in Fe5C(CO)15 and Fe4C(CO)13.[9]
24_2-.png)
Bonding
For low nuclearity clusters, bonding is often described as if it is localized. For this purpose, the eighteen electron rule is used. Thus, 34 electrons in an organometallic omplex predicts a dimetallic complex with a metal-metal bond. For higher nuclearity clusters, more elaborate rules are invoked including Jemmis mno rules and Polyhedral skeletal electron pair theory.
Although clusters are often written with discrete M-M bonds, the nature of this bonding is unclear, especially when there are bridging ligands.[11]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Paul J. Dyson, J. Scott McIndoe "Transition Metal Carbonyl Cluster Chemistry" Taylor & Francis, 2000.
- 1 2 3 Bhaduri, S.; Sharma, K.; Mukesh, D. Proc. Indian Acad. Sci. 1994, 713-716.
- 1 2 Bhaduri ,S. Current Science, 2000, 78(11), 1318-1324
- ↑ Calabrese, J. C.; Dahl, L. F.; Chini, P.; Longoni, G.; Martinengo, S. J. Am. Chem. Soc., 1974, 96 (8), pp 2614–2616
- ↑ Treguer, M.; Remita, H.; Pernot, P.; Khatouri, J.; Belloni, J. J. Phys. Chem. A 2001, 105, 6102.
- ↑ S. Martinengo, D. Strumolo, P. Chini, "Dipotassium μ6-Carbido-Nona-μ-Carbonyl-Hexacarbonylhexarhodate(2-) K2[Rh6(CO)6(μ-CO)9-μ-C]" Inorganic Syntheses, 1980, Volume 20, Pages: 212–215, 2007. doi:10.1002/9780470132517.ch48
- ↑ Elena Cariati, Claudia Dragonetti, Elena Lucenti, Dominique Roberto, "Tri- and Hexaruthnium Carbonyl Clusters" Inorganic Syntheses, 2004, Volume 35, 210.
- ↑ Ernestine W. Hill, John S. Bradley, "Tetrairon Carbido Carbonyl Clusters" Inorganic Syntheses, 1990, Volume 27, Pages: 182–188. doi:10.1002/9780470132586.ch36
- ↑ Jackson, P. F., Johnson, B. F. G., Lewis, J., Nelson, W. J. H., McPartlin, M., "The synthesis of the cluster dianion [Os10C(CO)24]2- by pyrolysis. X-Ray structure analysis of [N(PPh3)2]2[Os10C(CO)24] and [Os5C(CO)14H(NC5H4)]", Journal of the Chemical Society, Dalton Transactions 1982, 2099.doi:10.1039/DT9820002099
- ↑ Jennifer C. Green, Malcolm L. H. Green, Gerard Parkin "The occurrence and representation of three-centre two-electron bonds in covalent inorganic compounds" Chem. Commun. 2012, 11481-11503. doi:10.1039/c2cc35304k