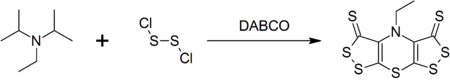N,N-Diisopropylethylamine
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N-Ethyl-N-(propan-2-yl)propan-2-amine | |
Other names
| |
| Identifiers | |
| 7087-68-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 73565 |
| ECHA InfoCard | 100.027.629 |
| EC Number | 230-392-0 |
| MeSH | N,N-diisopropylethylamine |
| PubChem | 81531 |
| UN number | 2733 |
| |
| |
| Properties | |
| C8H19N | |
| Molar mass | 129.25 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| Density | 0.742 g mL−1 |
| Melting point | −50 to −46 °C (−58 to −51 °F; 223 to 227 K) |
| Boiling point | 126.6 °C; 259.8 °F; 399.7 K |
| Vapor pressure | 4.1 kPa (at 37.70 °C) |
| Refractive index (nD) |
1.414 |
| Hazards | |
| GHS pictograms |    |
| GHS signal word | DANGER |
| H225, H301, H314, H412 | |
| P210, P273, P280, P301+310, P305+351+338, P310 | |
| EU classification (DSD) |
|
| R-phrases | R11, R22, R34, R52/53 |
| S-phrases | S16, S26, S36/37/39 |
| Flash point | 10 °C (50 °F; 283 K) |
| Explosive limits | 0.7–6.3% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
200–500 mg kg−1 (oral, rat) |
| Related compounds | |
| Related amines |
|
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
N,N-Diisopropylethylamine, or Hünig's base, DIPEA or DIEA, is an organic compound and an amine. It is used in organic chemistry as a base. Because the nitrogen atom is shielded by the two isopropyl groups and an ethyl group only a proton is small enough to easily fit. Like 2,2,6,6-tetramethylpiperidine, this compound is a good base but a poor nucleophile, which makes it a useful organic reagent.[1] Hünig's base is named after the German chemist Siegfried Hünig. The compound is a colourless liquid.
Hünig's base is commercially available. It is traditionally prepared by the alkylation of diisopropylamine with diethyl sulfate.[2] If necessary, the compound can be purified by distillation from potassium hydroxide.
Reactions
Non-nucleophilic base
Hünig's base was investigated for its use as a selective reagent in the alkylation of secondary amines to tertiary amines by alkyl halides. This organic reaction is often hampered by a quaternization reaction to the quaternary ammonium salt but this side-reaction is absent when Hünig's base is present.[3]
Synthesis of scorpionine
Hünig's base forms a complex heterocyclic compound called scorpionine by a reaction with disulfur dichloride catalyzed by DABCO in a one-pot synthesis.[4]
References
- ↑ Sorgi, K. L. (2001). "Diisopropylethylamine". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd254.
- ↑ Hünig, S.; Kiessel, M. (1958). "Spezifische Protonenacceptoren als Hilfsbasen bei Alkylierungs- und Dehydrohalogenierungsreaktionen". Chemische Berichte. 91 (2): 380–392. doi:10.1002/cber.19580910223.
- ↑ Moore, J. L.; Taylor, S. M.; Soloshonok, V. A. (2005). "An efficient and operationally convenient general synthesis of tertiary amines by direct alkylation of secondary amines with alkyl halides in the presence of Huenig's base". Arkivoc. 2005 (part vi): 287–292. EJ-1549C.
- ↑ Rees, W.; Marcos, C. F.; Polo, C.; Torroba, T.; Rakitin O. A. (1997). "From Hünig's Base to Bis([1,2]dithiolo)-[1,4]thiazines in One Pot: The Fast Route to Highly Sulfurated Heterocycles". Angewandte Chemie International Edition. 36 (3): 281–283. doi:10.1002/anie.199702811.