p-Hydroxynorephedrine
 | |
| Identifiers | |
|---|---|
| |
| Synonyms |
4-Hydroxynorephedrine para-Hydroxynorephedrine |
| CAS Number |
552-85-2 |
| PubChem (CID) | 11099 |
| ChemSpider |
10628 |
| Chemical and physical data | |
| Formula | C9H13NO2 |
| Molar mass | 167.21 g/mol |
| |
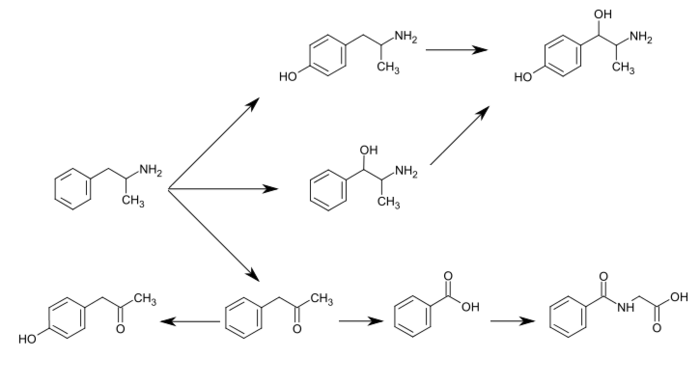
p-Hydroxynorephedrine, or 4-hydroxynorephedrine, is the para-hydroxy analog of norephedrine and an active sympathomimetic metabolite of amphetamine in humans.[1][2] When it occurs as a metabolite of amphetamine, it is produced from both p-hydroxyamphetamine and norephedrine.[2][3][4]
Amphetamine metabolism
Metabolic pathways of amphetamine in humans[sources 1]
See also
Reference notes
References
- ↑ "p-Hydroxynorephedrine". NCBI. PubChem Compound. Retrieved 25 October 2013.
- 1 2 "Adderall XR Prescribing Information" (PDF). Medication Guide. United States Food and Drug Administration. Retrieved 7 October 2013.
- ↑ "Amphetamine: Biomedical Effects and Toxicity". NCBI. Pubchem Compound. Retrieved 12 October 2013.
- ↑ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". J. Pharm. Biomed. Anal. 30 (2): 247–55. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ↑ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ↑ Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W. Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450. Retrieved 11 September 2015.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ↑ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). J. Biol. Chem. 249 (2): 454–458. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ↑ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circ. Res. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201.
Subjects with exceptionally low levels of serum dopamine-β-hydroxylase activity showed normal cardiovascular function and normal β-hydroxylation of an administered synthetic substrate, hydroxyamphetamine.
- ↑ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacol. Ther. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602
 . PMID 15922018.
. PMID 15922018.
"Table 5: N-containing drugs and xenobiotics oxygenated by FMO" - ↑ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". J. Pharmacol. Exp. Ther. 288 (3): 1251–1260. PMID 10027866.
- ↑ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". J. Pharm. Biomed. Anal. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ↑ "Substrate/Product". butyrate-CoA ligase. BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014.
- ↑ "Substrate/Product". glycine N-acyltransferase. BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014.
External links
- p-Hydroxynorephedrine at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia - version of the 11/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.