TAAR1
| TAAR1 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||
| Aliases | TAAR1, TA1, TAR1, TRAR1, trace amine associated receptor 1 | ||||||||||||||||
| External IDs | MGI: 2148258 HomoloGene: 24938 GeneCards: TAAR1 | ||||||||||||||||
| Targeted by Drug | |||||||||||||||||
| phenethylamine, dextroamphetamine, octopamine, R(-)amphetamine, RO5166017, tyramine, EPPTB, 2-phenylpropylamine, dopamine, amphetamine[1] | |||||||||||||||||
| |||||||||||||||||
| RNA expression pattern | |||||||||||||||||
 | |||||||||||||||||
| More reference expression data | |||||||||||||||||
| Orthologs | |||||||||||||||||
| Species | Human | Mouse | |||||||||||||||
| Entrez | |||||||||||||||||
| Ensembl | |||||||||||||||||
| UniProt | |||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||
| RefSeq (protein) | |||||||||||||||||
| Location (UCSC) | Chr 6: 132.64 – 132.65 Mb | Chr 10: 23.92 – 23.92 Mb | |||||||||||||||
| PubMed search | [2] | [3] | |||||||||||||||
| Wikidata | |||||||||||||||||
| View/Edit Human | View/Edit Mouse |
Trace amine-associated receptor 1 (TAAR1) is a protein that in humans is encoded by the TAAR1 gene.[4] TAAR1 is an amine-activated Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) that is primarily located in several peripheral organs, lymphocytes, astrocytes, and in the intracellular compartments within the presynaptic plasma membrane (i.e., axon terminal) of monoamine neurons in the central nervous system (CNS).[5][6][7] TAAR1 was discovered in 2001 by two independent groups of investigators, Borowski et al. and Bunzow et al.[8][9] TAAR1 is one of six functional trace amine-associated receptors in humans, which are so named for their ability to bind endogenous amines that occur in tissues at trace concentrations.[10][11] TAAR1 plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS;[6][10] it also affects immune system and neuroimmune system function through different mechanisms.[12][13][14][15]
The primary endogenous ligands of the human TAAR1 receptor, by rank order of potency, are:
tyramine > β-phenethylamine > dopamine = octopamine.[5]
Discovery
TAAR1 was discovered independently by Borowski et al. and Bunzow et al. in 2001. To find the genetic variants responsible for TAAR1 synthesis, they used mixtures of oligonucleotides with sequences related to G protein-coupled receptors (GPCRs) of serotonin and dopamine to discover novel DNA sequences in rat genomic DNA and cDNA, which they then amplified and cloned. The resulting sequence was not found in any database and coded for TAAR1.[8][9]
Structure
TAAR1 shares structural similarities with the class A rhodopsin GPCR subfamily.[9] It has 7 transmembrane domains with short N and C terminal extensions.[16] TAAR1 is 62–96% identical with TAARs2-15, which suggests that the TAAR subfamily has recently evolved; while at the same time, the low degree of similarity between TAAR1 orthologues suggests that they are rapidly evolving.[8] TAAR1 shares a predictive peptide motif with all other TAARs. This motif overlaps with transmembrane domain VII, and its identity is NSXXNPXX[Y,H]XXX[Y,F]XWF. TAAR1 and its homologues have ligand pocket vectors that utilize a sets of 35 amino acids known to be involved directly in receptor-ligand interaction.[11]
Gene
All TAAR genes are located on a single chromosome spanning 109kb of human chromosome 6q23.1, 192 kb of mouse chromosome 10A4, and 216 kb of rat chromosome 1p12. Each TAAR is derived from a single exon, except for TAAR2, which is coded by two exons.[11]
Tissue distribution
To date, TAAR1 has been identified and cloned in five different mammal genomes: human, mouse, rat, monkey, and chimpanzee. In rats, mRNA for TAAR1 is found at low to moderate levels in peripheral tissues like the stomach, kidney, and lungs, and at low levels in the brain.[8] Rhesus monkey Taar1 and human TAAR1 (hTAAR1) share high sequence similarity, and TAAR1 mRNA is highly expressed in the same important monoaminergic regions of both species. These regions include the dorsal and ventral caudate nucleus, putamen, substantia nigra, nucleus accumbens, ventral tegmental area, locus coeruleus, amygdala, and raphe nucleus.[5][17] TAAR1 has also been identified in human astrocytes.[5][12]
TAAR1 is the only TAAR subtype not found in the olfactory epithelium.[18]
Location within neurons
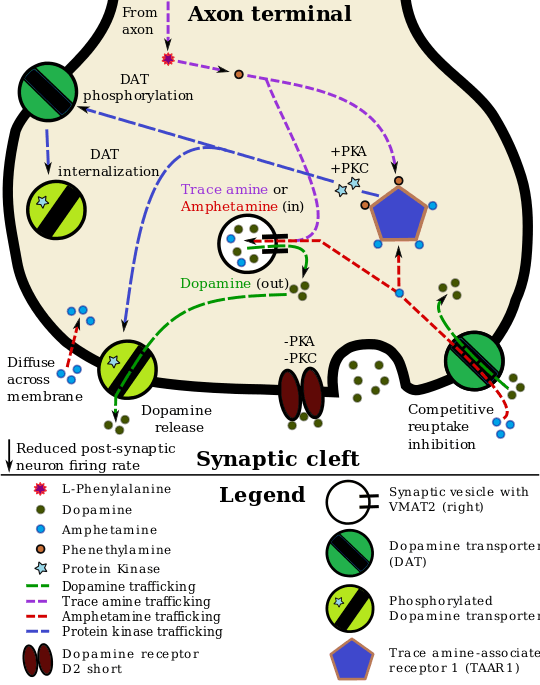
Human TAAR1 is an intracellular receptor expressed within the presynaptic terminal of monoamine neurons.[6][10][19] In model cell systems, hTAAR1 has extremely poor membrane expression.[19] A method to induce hTAAR1 membrane expression has been used to study its pharmacology via a bioluminescence resonance energy transfer cAMP assay.[19]
Because TAAR1 is an intracellular receptor in monoamine neurons, TAAR1 ligands must enter the presynaptic neuron through a membrane transport protein[note 1] or be able to diffuse across the presynaptic membrane in order to reach the receptor and produce reuptake inhibition and neurotransmitter efflux.[10] Consequently, the efficacy of a particular TAAR1 ligand in producing these effects in different monoamine neurons is a function of both its binding affinity at TAAR1 and its capacity to move across the presynaptic membrane at each type of neuron.[10] The variability between a TAAR1 ligand's substrate affinity at the various monoamine transporters accounts for much of the difference in its capacity to produce neurotransmitter release and reuptake inhibition in different types of monoamine neurons.[10] E.g., a TAAR1 ligand which can easily pass through the norepinephrine transporter, but not the serotonin transporter, will produce – all else equal – markedly greater TAAR1-induced effects in norepinephrine neurons as compared to serotonin neurons.
Receptor oligomers
TAAR1 forms GPCR oligomers with monoamine autoreceptors in neurons in vivo.[20][21] These hetero-oligomers include:
Ligands
Agonists
Trace amines
Trace amines are endogenous amines which act as agonists at TAAR1 and are present in extracellular concentrations of 0.1–10 nM in the brain, constituting less than 1% of total biogenic amines in the mammalian nervous system.[23] Some of the human trace amines include tryptamine, phenethylamine (PEA), N-methylphenethylamine, p-tyramine, m-tyramine, N-methyltyramine, p-octopamine, m-octopamine, and synephrine. These share structural similarities with the three common monoamines: serotonin, dopamine, and norepinephrine. Each ligand has a different potency, measured as increases cyclic AMP (cAMP) concentration after the binding event.
The rank order of potency for the primary endogenous ligands at hTAAR1 is:
tyramine > β-phenethylamine > dopamine = octopamine.[5]
Thyronamines
Thyronamines are molecular derivatives of the thyroid hormone and are very important for endocrine system function. 3-Iodothyronamine (T1AM) is the most potent TAAR1 agonist yet discovered, although it lacks monoamine transporter affinity and therefore has little effect in monoamine neurons of the central nervous system. Activation of TAAR1 by T1AM results in the production of large amounts of cAMP. This effect is coupled with decreased body temperature and cardiac output.
Synthetic
- Amphetamine and the amphetamine-related compounds methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA), and 2,5-dimethoxy-4-iodoamphetamine (DOI) are all potent rat TAAR1 (rTAAR1) agonists. Upon association with TAAR1, they elicit increases in cAMP production similar to those of PEA and p-tyramine. Not surprisingly, these amphetamine-like compounds are structurally similar to PEA and p-tyramine.[9][24]
- Benzofurans: 5-APB, 5-APDB, 6-APB, 6-APDB, 4-APB, 7-APB, 5-EAPB, and 5-MAPDB, as well as the benzodifuran 2C-B-FLY, are hTAAR1 agonists that have an MDMA-like pharmacodynamic profile.[25]
- The methylphenethylamines are agonists of hTAAR1; these include α-methylphenethylamine (amphetamine), β-methylphenethylamine, N-methylphenethylamine (a trace amine), 2-methylphenethylamine, 3-methylphenethylamine, and 4-methylphenethylamine.[26]
- In rats, lysergic acid diethylamide (LSD) is an agonist of rTAAR1,[9] but in humans it lacks any affinity for hTAAR1.[26]
- Certain 2-aminooxazoline compounds (RO5166017, RO5256390, RO5203648, and RO5263397) are orally bioavailable, highly potent, and selective agonists of TAAR1 in laboratory animals.[27]
- RO5166017 or (S)-4-[(ethylphenylamino)methyl]-4,5-dihydrooxazol-2-ylamine is a selective TAAR1 agonist without significant activity at other targets.[28]
- RO5203648 and RO5263397 are highly selective TAAR1 partial agonists.[20] RO5203648 demonstrated clear antidepressant and anti-psychotic activity, additionally it attenuated drug self-administration and exhibited wakefulness promoting and cognition enhancing properties in murine and simian models.[29]
Antagonists
- EPPTB or N-(3-ethoxyphenyl)-4-(pyrrolidin-1-yl)-3-trifluoromethylbenzamide is a selective TAAR1 antagonist.[30]
Function
Monoaminergic systems
Before the discovery of TAAR1, trace amines were believed to serve very limited functions. They were thought to induce noradrenaline release from sympathetic nerve endings and compete for catecholamine or serotonin binding sites on cognate receptors, transporters, and storage sites.[23] Today, they are believed to play a much more dynamic role by regulating monoaminergic systems in the brain.
One of the downstream effects of active TAAR1 is to increase cAMP in the presynaptic cell via Gαs G-protein activation of adenylyl cyclase.[8][9][11] This alone can have a multitude of cellular consequences. A main function of the cAMP may be to up-regulate the expression of trace amines in the cell cytoplasm.[24] These amines would then activate intracellular TAAR1. Monoamine autoreceptors (e.g., D2 short, presynaptic α2, and presynaptic 5-HT1A) have the opposite effect of TAAR1, and together these receptors provide a regulatory system for monoamines.[10] Notably, amphetamine and trace amines bind to TAAR1, but not monoamine autoreceptors.[10] The effect of TAAR1 agonists on monoamine transporters in the brain appears to be site-specific.[10] Imaging studies indicate that monoamine reuptake inhibition by amphetamine and trace amines is dependent upon the presence of TAAR1 co-localization in the associated monoamine neurons.[10] As of 2010, co-localization of TAAR1 and the dopamine transporter (DAT) has been visualized in rhesus monkeys, but co-localization of TAAR1 with the norepinephrine transporter (NET) and the serotonin transporter (SERT) has only been evidenced by messenger RNA (mRNA) expression.[10]
In neurons with co-localized TAAR1, TAAR1 agonists increase the concentrations of the associated monoamines in the synaptic cleft, thereby increasing post-synaptic receptor binding.[10] Through direct activation of G protein-coupled inwardly-rectifying potassium channels (GIRKs), TAAR1 can reduce the firing rate of dopamine neurons, in turn preventing a hyper-dopaminergic state.[28][31][32] Amphetamine and trace amines can enter the presynaptic neuron either through DAT or by diffusing across the neuronal membrane directly.[10] As a consequence of DAT uptake, amphetamine and trace amines produce competitive reuptake inhibition at the transporter.[10] Upon entering the presynaptic neuron, these compounds activate TAAR1 which, through protein kinase A (PKA) and protein kinase C (PKC) signaling, causes DAT phosphorylation. Phosphorylation by either protein kinase can result in DAT internalization (non-competitive reuptake inhibition), but PKC-mediated phosphorylation alone induces reverse transporter function (dopamine efflux).[10][33]
Immune system
Expression of TAAR1 on lymphocytes is associated with activation of lymphocyte immuno-characteristics.[14] In the immune system, TAAR1 transmits signals through active PKA and PKC phosphorylation cascades.[14] In a recent study, Panas et al. observed that methamphetamine had these effects, suggesting that, in addition to brain monoamine regulation, amphetamine-related compounds may have an effect on the immune system.[14] A recent paper showed that, along with TAAR1, TAAR2 is required for full activity of trace amines in PMN cells.[15]
Phytohaemagglutinin upregulates hTAAR1 mRNA in circulating leukocytes;[5] in these cells, TAAR1 activation mediates leukocyte chemotaxis toward TAAR1 agonists.[5] TAAR1 agonists (specifically, trace amines) have also been shown to induce interleukin 4 secretion in T-cells and immunoglobulin E (IgE) secretion in B cells.[5]
Astrocyte-localized TAAR1 regulates EAAT2 levels and function in these cells;[12] this has been implicated in methamphetamine-induced pathologies of the neuroimmune system.[12]
Clinical significance
Low phenethylamine (PEA) concentration in the brain is associated with major depressive disorder,[8][23][34] and high concentrations are associated with schizophrenia.[34][35] Low PEA levels and under-activation of TAAR1 also appears to be associated with ADHD.[34][35][36] It is hypothesized that insufficient PEA levels result in TAAR1 inactivation and overzealous monoamine uptake by transporters, possibly resulting in depression.[8][23] Some antidepressants function by inhibiting monoamine oxidase (MAO), which increases the concentration of trace amines, which is speculated to increase TAAR1 activation in presynaptic cells.[8][11] Decreased PEA metabolism has been linked to schizophrenia, a logical finding considering excess PEA would result in over-activation of TAAR1 and prevention of monoamine transporter function. Interestingly, mutations in region q23.1 of human chromosome 6 – the same chromosome that codes for TAAR1 – have been linked to schizophrenia.[11]
Medical reviews from February 2015 and 2016 noted that TAAR1-selective ligands have significant therapeutic potential for treating psychostimulant addictions (e.g., cocaine, amphetamine, methamphetamine, etc.).[6][7]
Research
A large candidate gene association study published in September 2011 found significant differences in TAAR1 allele frequencies between a cohort of fibromyalgia patients and a chronic pain-free control group, suggesting this gene may play an important role in the pathophysiology of the condition; this possibly presents a target for therapeutic intervention.[37]
In preclinical research on rats, TAAR1 activation in pancreatic cells promotes insulin, peptide YY, and GLP-1 secretion;[38] therefore, TAAR1 is potentially a biological target for the treatment of obesity and diabetes.[38]
Notes
- ↑ In dopamine, norepinephrine, and serotonin neurons, the primary membrane transporters are DAT, NET, and SERT respectively.[10]
- ↑ TAAR1–D2sh is a presynaptic heterodimer which involves the relocation of TAAR1 from the intracellular space to D2sh at the plasma membrane, increased D2sh agonist binding affinity, and signal transduction through the calcium–PKC–NFAT pathway and G-protein independent PKB–GSK3 pathway.[6][22]
References
- ↑ "Drugs that physically interact with Trace amine-associated receptor 1 view/edit references on wikidata".
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: TAAR1 trace amine associated receptor 1".
- 1 2 3 4 5 6 7 8 Maguire JJ, Davenport AP (19 July 2016). "Trace amine receptor: TA1 receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Retrieved 22 September 2016.
- 1 2 3 4 5 Grandy DK, Miller GM, Li JX (February 2016). ""TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference". Drug Alcohol Depend. 159: 9–16. doi:10.1016/j.drugalcdep.2015.11.014. PMID 26644139.
This original observation of TAAR1 and DA D2R interaction has subsequently been confirmed and expanded upon with observations that both receptors can heterodimerize with each other under certain conditions ... Additional DA D2R/TAAR1 interactions with functional consequences are revealed by the results of experiments demonstrating that in addition to the cAMP/PKA pathway (Panas et al., 2012) stimulation of TAAR1-mediated signaling is linked to activation of the Ca++/PKC/NFAT pathway (Panas et al.,2012) and the DA D2R-coupled, G protein-independent AKT/GSK3 signaling pathway (Espinoza et al., 2015; Harmeier et al., 2015), such that concurrent TAAR1 and DA DR2R activation could result in diminished signaling in one pathway (e.g. cAMP/PKA) but retention of signaling through another (e.g., Ca++/PKC/NFA)
- 1 2 Jing L, Li JX (August 2015). "Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction". Eur. J. Pharmacol. 761: 345–352. doi:10.1016/j.ejphar.2015.06.019. PMID 26092759.
TAAR1 is largely located in the intracellular compartments both in neurons (Miller, 2011), in glial cells (Cisneros and Ghorpade, 2014) and in peripheral tissues (Grandy, 2007) ... Taken together,the data reviewed here strongly support that TAAR1 is implicated in the functional regulation of monoaminergic systems, especially dopaminergic system, and that TAAR1 serves as a homeostatic “brake” system that is involved in the modulation of dopaminergic activity. Existing data provided robust preclinical evidence supporting the development of TAAR1 agonists as potential treatment for psychostimulant abuse and addiction. ... Given that TAAR1 is primarily located in the intracellular compartments and existing TAAR1 agonists are proposed to get access to the receptors by translocation to the cell interior (Miller, 2011), future drug design and development efforts may need to take strategies of drug delivery into consideration (Rajendran et al., 2010).
- 1 2 3 4 5 6 7 8 Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C (July 2001). "Trace amines: identification of a family of mammalian G protein-coupled receptors". Proceedings of the National Academy of Sciences of the United States of America. 98 (16): 8966–8971. doi:10.1073/pnas.151105198. PMC 55357
 . PMID 11459929.
. PMID 11459929. - 1 2 3 4 5 6 Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK (December 2001). "Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor". Molecular Pharmacology. 60 (6): 1181–1188. doi:10.1124/mol.60.6.1181. PMID 11723224.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101
 . PMID 21073468.
. PMID 21073468. - 1 2 3 4 5 6 Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC (March 2005). "Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors". Genomics. 85 (3): 372–385. doi:10.1016/j.ygeno.2004.11.010. PMID 15718104.
- 1 2 3 4 Cisneros IE, Ghorpade A (October 2014). "Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes". Neuropharmacology. 85: 499–507. doi:10.1016/j.neuropharm.2014.06.011. PMID 24950453.
TAAR1 overexpression significantly decreased EAAT-2 levels and glutamate clearance ... METH treatment activated TAAR1 leading to intracellular cAMP in human astrocytes and modulated glutamate clearance abilities. Furthermore, molecular alterations in astrocyte TAAR1 levels correspond to changes in astrocyte EAAT-2 levels and function.
- ↑ Rogers TJ (2012). "The molecular basis for neuroimmune receptor signaling". J Neuroimmune Pharmacol. 7 (4): 722–724. doi:10.1007/s11481-012-9398-4. PMC 4011130
 . PMID 22935971.
. PMID 22935971. - 1 2 3 4 Panas MW, Xie Z, Panas HN, Hoener MC, Vallender EJ, Miller GM (December 2012). "Trace amine associated receptor 1 signaling in activated lymphocytes". Journal of Neuroimmune Pharmacology. 7 (4): 866–876. doi:10.1007/s11481-011-9321-4. PMC 3593117
 . PMID 22038157.
. PMID 22038157. - 1 2 Babusyte A, Kotthoff M, Fiedler J, Krautwurst D (March 2013). "Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2". Journal of Leukocyte Biology. 93 (3): 387–394. doi:10.1189/jlb.0912433. PMID 23315425.
- ↑ Xie Z, Miller GM (November 2009). "Trace amine-associated receptor 1 as a monoaminergic modulator in brain". Biochemical Pharmacology. 78 (9): 1095–1104. doi:10.1016/j.bcp.2009.05.031. PMC 2748138
 . PMID 19482011.
. PMID 19482011. - ↑ Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, Miller GM (April 2007). "Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro". The Journal of Pharmacology and Experimental Therapeutics. 321 (1): 116–127. doi:10.1124/jpet.106.116863. PMID 17234900.
- ↑ Liberles SD, Buck LB (August 2006). "A second class of chemosensory receptors in the olfactory epithelium". Nature. 442 (7103): 645–650. doi:10.1038/nature05066. PMID 16878137.
- 1 2 3 Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, Violin JD, Lefkowitz RJ, Caron MG, Gainetdinov RR (September 2008). "Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor". Molecular Pharmacology. 74 (3): 585–594. doi:10.1124/mol.108.048884. PMC 3766527
 . PMID 18524885.
. PMID 18524885. - 1 2 3 Lam VM, Espinoza S, Gerasimov AS, Gainetdinov RR, Salahpour A (June 2015). "In-vivo pharmacology of Trace-Amine Associated Receptor 1". Eur. J. Pharmacol. doi:10.1016/j.ejphar.2015.06.026. PMID 26093041.
- 1 2 Dinter J, Mühlhaus J, Jacobi SF, Wienchol CL, Cöster M, Meister J, Hoefig CS, Müller A, Köhrle J, Grüters A, Krude H, Mittag J, Schöneberg T, Kleinau G, Biebermann H (June 2015). "3-iodothyronamine differentially modulates α-2A-adrenergic receptor-mediated signaling". J. Mol. Endocrinol. 54 (3): 205–216. doi:10.1530/JME-15-0003. PMID 25878061.
Moreover, in ADRA2A/TAAR1 hetero-oligomers, the capacity of NorEpi to stimulate Gi/o signaling is reduced by co-stimulation with 3-T1AM. The present study therefore points to a complex spectrum of signaling modification mediated by 3-T1AM at different G protein-coupled receptors.
- ↑ Harmeier A, Obermueller S, Meyer CA, Revel FG, Buchy D, Chaboz S, Dernick G, Wettstein JG, Iglesias A, Rolink A, Bettler B, Hoener MC (2015). "Trace amine-associated receptor 1 activation silences GSK3β signaling of TAAR1 and D2R heteromers". Eur Neuropsychopharmacol. 25 (11): 2049–2061. doi:10.1016/j.euroneuro.2015.08.011. PMID 26372541.
Interaction of TAAR1 with D2R altered the subcellular localization of TAAR1 and increased D2R agonist binding affinity.
- 1 2 3 4 Zucchi R, Chiellini G, Scanlan TS, Grandy DK (December 2006). "Trace amine-associated receptors and their ligands". British Journal of Pharmacology. 149 (8): 967–978. doi:10.1038/sj.bjp.0706948. PMC 2014643
 . PMID 17088868.
. PMID 17088868. Other biogenic amines are present in the central nervous system at very low concentrations in the order of 0.1–10 nm, representing <1% of total biogenic amines (Berry, 2004). For these compounds, the term ‘trace amines' was introduced. Although somewhat loosely defined, the molecules generally considered to be trace amines include para-tyramine, meta-tyramine, tryptamine, β-phenylethylamine, para-octopamine and meta-octopamine (Berry, 2004) (Figure 2).
- 1 2 Xie Z, Miller GM (July 2009). "A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain". The Journal of Pharmacology and Experimental Therapeutics. 330 (1): 316–325. doi:10.1124/jpet.109.153775. PMC 2700171
 . PMID 19364908.
. PMID 19364908. - ↑ Rickli A, Kopf S, Hoener MC, Liechti ME (July 2015). "Pharmacological profile of novel psychoactive benzofurans". British Journal of Pharmacology. 172 (13): 3412–3425. doi:10.1111/bph.13128. PMID 25765500.
- 1 2 Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL (January 2007). "Pharmacologic characterization of the cloned human trace amine-associated receptor1 (TAAR1) and evidence for species differences with the rat TAAR1". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 475–485. doi:10.1124/jpet.106.112532. PMID 17038507.
- ↑ Galley G, Beurier A, Décoret G, Goergler A, Hutter R, Mohr S, Pähler A, Schmid P, Türck D, Unger R, Zbinden KG, Hoener MC, Norcross RD (2016). "Discovery and Characterization of 2-Aminooxazolines as Highly Potent, Selective, and Orally Active TAAR1 Agonists". ACS Med Chem Lett. 7 (2): 192–197. doi:10.1021/acsmedchemlett.5b00449. PMID 26985297.
- 1 2 Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC (May 2011). "TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity". Proc. Natl. Acad. Sci. U.S.A. 108 (20): 8485–8490. doi:10.1073/pnas.1103029108. PMC 3101002
 . PMID 21525407.
. PMID 21525407. - ↑ Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velázquez-Sánchez C, Sotnikova TD, Morairty SR, Harmeier A, Groebke Zbinden K, Norcross RD, Bradaia A, Kilduff TS, Biemans B, Pouzet B, Caron MG, Canales JJ, Wallace TL, Wettstein JG, Hoener MC (June 2012). "Trace Amine-Associated Receptor 1 Partial Agonism Reveals Novel Paradigm for Neuropsychiatric Therapeutics". Biol Psychiatry. 72 (11): 934–942. doi:10.1016/j.biopsych.2012.05.014. PMID 22705041.
- ↑ Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC, Bettler B (November 2009). "The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system". Proceedings of the National Academy of Sciences of the United States of America. 106 (47): 20081–20086. doi:10.1073/pnas.0906522106. PMC 2785295
 . PMID 19892733.
. PMID 19892733. - ↑ Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB (2011). "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Frontiers in Systems Neuroscience. 5: 56. doi:10.3389/fnsys.2011.00056. PMC 3131148
 . PMID 21772817.
. PMID 21772817. inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.
- ↑ mct (28 January 2012). "TAAR1". GenAtlas. University of Paris. Retrieved 29 May 2014.
" • tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)" - ↑ Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP (March 2009). "International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature". Pharmacological Reviews. 61 (1): 1–8. doi:10.1124/pr.109.001107. PMC 2830119
 . PMID 19325074.
. PMID 19325074. - 1 2 3 Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
The dysregulation of TA levels has been linked to several diseases, which highlights the corresponding members of the TAAR family as potential targets for drug development. In this article, we focus on the relevance of TAs and their receptors to nervous system-related disorders, namely schizophrenia and depression; however, TAs have also been linked to other diseases such as migraine, attention deficit hyperactivity disorder, substance abuse and eating disorders [7,8,36]. Clinical studies report increased β-PEA plasma levels in patients suffering from acute schizophrenia [37] and elevated urinary excretion of β-PEA in paranoid schizophrenics [38], which supports a role of TAs in schizophrenia. As a result of these studies, β-PEA has been referred to as the body’s ‘endogenous amphetamine’ [39]
- 1 2 Sotnikova TD, Caron MG, Gainetdinov RR (August 2009). "Trace amine-associated receptors as emerging therapeutic targets". Mol. Pharmacol. 76 (2): 229–235. doi:10.1124/mol.109.055970. PMC 2713119
 . PMID 19389919.
. PMID 19389919. Although the functional role of trace amines in mammals remains largely enigmatic, it has been noted that trace amine levels can be altered in various human disorders, including schizophrenia, Parkinson's disease, attention deficit hyperactivity disorder (ADHD), Tourette syndrome, and phenylketonuria (Boulton, 1980; Sandler et al., 1980). It was generally held that trace amines affect the monoamine system indirectly via interaction with plasma membrane transporters [such as plasma membrane dopamine transporter (DAT)] and vesicular storage (Premont et al., 2001; Branchek and Blackburn, 2003; Berry, 2004; Sotnikova et al., 2004). ...
Furthermore, DAT-deficient mice provide a model to investigate the inhibitory actions of amphetamines on hyperactivity, the feature of amphetamines believed to be important for their therapeutic action in ADHD (Gainetdinov et al., 1999; Gainetdinov and Caron, 2003). It should be noted also that the best-established agonist of TAAR1, β-PEA, shared the ability of amphetamine to induce inhibition of dopamine-dependent hyperactivity of DAT-KO mice (Gainetdinov et al., 1999; Sotnikova et al., 2004).
Furthermore, if TAAR1 could be proven as a mediator of some of amphetamine's actions in vivo, the development of novel TAAR1-selective agonists and antagonists could provide a new approach for the treatment of amphetamine-related conditions such as addiction and/or disorders in which amphetamine is used therapeutically. In particular, because amphetamine has remained the most effective pharmacological treatment in ADHD for many years, a potential role of TAAR1 in the mechanism of the “paradoxical” effectiveness of amphetamine in this disorder should be explored. - ↑ Berry MD (January 2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Rev Recent Clin Trials. 2 (1): 3–19. doi:10.2174/157488707779318107. PMID 18473983.
changes in trace amines, in particular PE, have been identified as a possible factor for the onset of attention deficit/hyperactivity disorder (ADHD) [5, 27, 43, 78]. PE has been shown to induce hyperactivity and aggression, two of the cardinal clinical features of ADHD, in experimental animals [100]. Hyperactivity is also a symptom of phenylketonuria, which as discussed above is associated with a markedly elevated PE turnover [44]. Further, amphetamines, which have clinical utility in ADHD, are good ligands at trace amine receptors [2]. Of possible relevance in this aspect is modafanil, which has shown beneficial effects in ADHD patients [101] and has been reported to enhance the activity of PE at TAAR1 [102]. Conversely, methylphenidate, which is also clinically useful in ADHD, showed poor efficacy at the TAAR1 receptor [2]. In this respect it is worth noting that the enhancement of functioning at TAAR1 seen with modafanil was not a result of a direct interaction with TAAR1 [102].
More direct evidence has been obtained recently for a role of trace amines in ADHD. Urinary PE levels have been reported to be decreased in ADHD patients in comparison to both controls and patients with autism [103-105]. Evidence for a decrease in PE levels in the brain of ADHD patients has also recently been reported [4]. In addition, decreases in the urine and plasma levels of the PE metabolite phenylacetic acid and the precursors phenylalanine and tyrosine have been reported along with decreases in plasma tyramine [103]. Following treatment with methylphenidate, patients who responded positively showed a normalization of urinary PE, whilst non-responders showed no change from baseline values [105]. - ↑ Smith SB, Maixner DW, Fillingim RB, Slade G, Gracely RH, Ambrose K, Zaykin DV, Hyde C, John S, Tan K, Maixner W, Diatchenko L (February 2012). "Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia". Arthritis and Rheumatism. 64 (2): 584–593. doi:10.1002/art.33338. PMC 3237946
 . PMID 21905019.
. PMID 21905019. - 1 2 Raab S, Wang H, Uhles S, Cole N, Alvarez-Sanchez R, Künnecke B, Ullmer C, Matile H, Bedoucha M, Norcross RD, Ottaway-Parker N, Perez-Tilve D, Conde Knape K, Tschöp MH, Hoener MC, Sewing S (2016). "Incretin-like effects of small molecule trace amine-associated receptor 1 agonists". Mol Metab. 5 (1): 47–56. doi:10.1016/j.molmet.2015.09.015. PMC 4703809
 . PMID 26844206.
. PMID 26844206.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.