Passivation (chemistry)
Passivation, in physical chemistry and engineering, refers to a material becoming "passive," that is, less affected or corroded by the environment of future use. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build from spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shell against corrosion. Passivation can occur only in certain conditions, and is used in microelectronics to enhance silicon.[1] The technique of passivation strengthens and preserves the appearance of metallics. In electrochemical treatment of water, passivation reduces the effectiveness of the treatment by increasing the circuit resistance, and active measures are typically used to overcome this effect, the most common being polarity reversal, which results in limited rejection of the fouling layer. Other proprietary systems to avoid electrode passivation, several discussed below, are the subject of ongoing research and development.
When exposed to air, many metals naturally form a hard, relatively inert surface, as in the tarnish of silver; others, like iron, corrosion to a somewhat rough surface by removal a substantial amount of metal, which either dissolves in the environment or reacts with it to produce a loosely adherent, porous coating of corrosion products. Corrosion coating reduces the rate of corrosion by varying degrees, depending on the kind of base metal and its environment, and is notably slower in room-temperature air for aluminium, chromium, zinc, titanium, and silicon (a metalloid); the shell of corrosion inhibits deeper corrosion, and operates as one form passivation. The inert surface layer, termed the ‘’native oxide layer‘’, is usually an oxide or a nitride, with a thickness of a monolayer (1-3 Å) for a noble metal such as platinum, about 15 Å for silicon, and nearer to 50 Å for aluminium after several years.[2][3][4]
Mechanisms
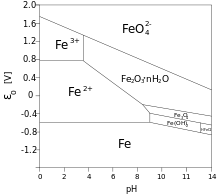
There has been much interest in determining the mechanisms that govern the increase of thickness of the oxide layer over time. Some of the important factors are the volume of oxide relative to the volume of the parent metal, the mechanism of oxygen diffusion through the metal oxide to the parent metal, and the relative chemical potential of the oxide. Boundaries between micro grains, if the oxide layer is crystalline, form an important pathway for oxygen to reach the unoxidized metal below. For this reason, vitreous oxide coatings – which lack grain boundaries – can retard oxidation.[6] The conditions necessary (but not sufficient) for passivation are recorded in Pourbaix diagrams. Some corrosion inhibitors help the formation of a passivation layer on the surface of the metals to which they are applied. Some compounds, dissolving in solutions (chromates, molybdates) form non-reactive and low solubility films on metal surfaces.
Discovery
In the mid 1800s, Christian Friedrich Schönbein discovered that when a piece of iron is placed in dilute nitric acid, it will dissolve and produce hydrogen, but if the iron is placed in concentrated nitric acid and then returned to the dilute nitric acid, little or no reaction will take place. Schönbein named the first state the active condition and the second the passive condition. If passive iron is touched by active iron, it becomes active again. In 1920, Ralph S. Lillie measured the effect of an active piece of iron touching a passive iron wire and found that "a wave of activation sweeps rapidly (at some hundred centimeters a second) over its whole length".[7][8]
Specific materials
Silicon
In the area of microelectronics, the formation of a strongly adhering passivating oxide is important to the performance of silicon.
In the area of photovoltaics, a passivating surface layer such as silicon nitride, silicon dioxide or titanium dioxide can reduce surface recombination - a significant loss mechanism in solar cells.
Aluminium
Pure aluminium naturally forms a thin surface layer of aluminium oxide on contact with oxygen in the atmosphere through a process called oxidation, which creates a physical barrier to corrosion or further oxidation in most environments. Aluminium alloys, however, offer little protection against corrosion. There are three main ways to passivate these alloys: alclading, chromate conversion coating and anodizing. Alclading is the process of metallurgically bonding a thin layer of pure aluminium to the aluminium alloy. Chromate conversion coating is a common way of passivating not only aluminum, but also zinc, cadmium, copper, silver, magnesium, and tin alloys. Anodizing forms a thick oxide coating. This finish is more robust than the other processes and also provides good electrical insulation, which the other two processes do not.
For example, prior to storing hydrogen peroxide in an aluminium container, the container can be passivated by rinsing it with a dilute solution of nitric acid and peroxide alternating with deionized water. The nitric acid and peroxide oxidizes and dissolves any impurities on the inner surface of the container, and the deionized water rinses away the acid and oxidized impurities.
Ferrous materials
Ferrous materials, including steel, may be somewhat protected by promoting oxidation ("rust") and then converting the oxidation to a metalophosphate by using phosphoric acid and further protected by surface coating. As the uncoated surface is water-soluble, a preferred method is to form manganese or zinc compounds by a process commonly known as Parkerizing or phosphate conversion. Older, less-effective but chemically-similar electrochemical conversion coatings included black oxidizing, historically known as bluing or browning. Ordinary steel forms a passivating layer in alkali environments, as reinforcing bar does in concrete.
Stainless steel
Stainless steels are corrosion-resistant by nature, which might suggest that passivating them would be unnecessary. However, stainless steels are not completely impervious to rusting. One common mode of corrosion in corrosion-resistant steels is when small spots on the surface begin to rust because grain boundaries or embedded bits of foreign matter (such as grinding swarf) allow water molecules to oxidize some of the iron in those spots despite the alloying chromium. This is called rouging. Some grades of stainless steel are especially resistant to rouging; parts made from them may therefore forgo any passivation step, depending on engineering decisions.[9]
Passivation processes are generally controlled by industry standards, the most prevalent among them today being ASTM A 967 and AMS 2700. These industry standards generally list several passivation processes that can be used, with the choice of specific method left to the customer and vendor. The "method" is either a nitric acid-based passivating bath, or a citric acid-based bath. The various 'types' listed under each method refer to differences in acid bath temperature and concentration. Sodium dichromate is often required as an additive to promote oxidation in certain 'types' of nitric-based acid baths.
Common among all of the different specifications and types are the following steps: Prior to passivation, the object must be cleaned of any contaminants and generally must undergo a validating test to prove that the surface is 'clean.' The object is then placed in an acidic passivating bath that meets the temperature and chemical requirements of the method and type specified between customer and vendor. (Temperatures can range from ambient to 140 degrees Fahrenheit, while minimum passivation times are usually 20 to 30 minutes). The parts are neutralized using a bath of aqueous sodium hydroxide, then rinsed with clean water and dried. The passive surface is validated using humidity, elevated temperature, a rusting agent (salt spray), or some combination of the three. However, proprietary passivation processes exist[10] for martensitic stainless steel, which is difficult to passivate, as microscopic discontinuities can form in the surface of a machined part during passivation in a typical nitric acid bath.[11] The passivation process removes exogenous iron,[12] creates/restores a passive oxide layer that prevents further oxidation (rust), and cleans the parts of dirt, scale, or other welding-generated compounds (e.g. oxides).[13][14]
It is not uncommon for some aerospace manufacturers to have additional guidelines and regulations when passivating their products that exceed the national standard. Often, these requirements will be cascaded down using Nadcap or some other accreditation system. Various testing methods are available to determine the passivation (or passive state) of stainless steel. The most common methods for validating the passivity of a part is some combination of high humidity and heat for a period of time, intended to induce rusting. Electro-chemical testers can also be utilized to commercially verify passivation.
Nickel
Nickel can be used for handling elemental fluorine, owing to the formation of a passivation layer of nickel fluoride. This fact is useful in water treatment and sewage treatment applications
See also
References
- ↑ IUPAC Goldbook
- ↑ http://www.semi1source.com/glossary/default.asp?searchterm=native+oxide
- ↑ O'M. Bockris 1977, p. 1325
- ↑ Fehlner, Francis P, Low-Temperature Oxidation:The Role of Vitreous Oxides, A Wiley-Interscience Publication, John Wiley & Sons, New York , 1986 ISBN 0471-87448-5
- ↑ University of Bath & Western Oregon University
- ↑ Fehlner, Francis P, ref.3.
- ↑ Lillie, Ralph S. (June 20, 1920). "The Recovery of Transmissivity in Passive Iron Wires as a Model of Recovery Processes in Irritable Living Systems". The Journal of General Physiology. Physiological Laboratory, Clark University, Worcester. 3 (2): 129–43. doi:10.1085/jgp.3.2.129. Retrieved 15 August 2015.
- ↑ Macinnes, Duncan A. (1939). The principles of electrochemistry. Reinnhold Publishing Corporation. pp. 447–451.
- ↑ "Stainless Steel Passivation". Arrow Cryogenics. Retrieved 28 February 2014.
- ↑ http://www.rpabrasives.com/services/passivation/passivation-process/
- ↑ http://www.cartech.com/techarticles.aspx?id=1566
- ↑ http://www.delstar.com/stainless-steel-passivation
- ↑ http://www.delstar.com/stainless-steel-passivation
- ↑ http://www.euro-inox.org/pdf/map/Passivating_Pickling_EN.pdf
Further reading
- ASTM (1 March 2010), ASTM A967: Standard specification for chemical passivation treatments for stainless steel parts (Rev 05e2 ed.), doi:10.1520/A0967-05E02. The most common commercial spec for passivation of stainless steel parts. Used in various industries; latest revision is active for new designs; legacy designs may still require older revisions or older standards, if the engineering has not been revisited.
- SAE (8 July 2011), AMS 2700: Passivation of corrosion resistant steels. (Rev D ed.). AMS specs are frequently used in the aerospace industry, and are sometimes stricter than other standards. Latest revision is active for new designs; legacy designs may still require older revisions or older standards, if the engineering has not been revisited.
- SAE (16 February 2005), AMS QQ-P-35: Passivation treatments for corrosion-resistant steel (Rev A ed.). AMS-QQ-P-35 superseded U.S. federal spec QQ-P-35 on 4 April 1997. AMS-QQ-P-35 itself was canceled and superseded in February 2005 by AMS 2700.
- U.S. government, QQ-P-35: Federal specification: Passivation treatments for corrosion-resistant steel (Rev C ed.). U.S. federal spec QQ-P-35 was superseded by AMS-QQ-P-35 on 4 April 1997 as part of the changeover instituted by the Perry memo. Both are now outdated; they are inactive for new designs, but legacy designs may still require their use, if the engineering has not been revisited.
- Chromate conversion coating (chemical film) per MIL-DTL-5541F for aluminium and aluminium alloy parts
- A standard overview on black oxide coatings is provided in MIL-HDBK-205, Phosphate & Black Oxide Coating of Ferrous Metals. Many of the specifics of Black Oxide coatings may be found in MIL-DTL-13924 (formerly MIL-C-13924). This Mil-Spec document additionally identifies various classes of Black Oxide coatings, for use in a variety of purposes for protecting ferrous metals against rust.
- Budinski, Kenneth G. (1988), Surface Engineering for Wear Resistance, Englewood Cliffs, New Jersey: Prentice Hall, p. 48.
- Brimi, Marjorie A. (1965), Electrofinishing, New York, New York: American Elsevier Publishing Company, Inc, pp. 62–63.
- Bockris, John O'M.; Reddy, Amulya K. N. (1977), Modern Electrochemistry: An Introduction to an Interdisciplinary Area, 2, Plenum Press, ISBN 0-306-25002-0.
- Passivisation : Debate over Paintability http://www.coilworld.com/5-6_12/rlw3.htm