Pourbaix diagram
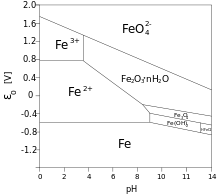
In chemistry, a Pourbaix diagram, also known as a potential/pH diagram, EH-pH diagram or a pE/pH diagram, maps out possible stable (equilibrium) phases of an aqueous electrochemical system. Predominant ion boundaries are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes. Similarly to phase diagrams, they do not allow for reaction rate or kinetic effects.
The diagrams are named after Marcel Pourbaix (1904–1998), the Russian-born, Belgian chemist who invented them.
Diagram
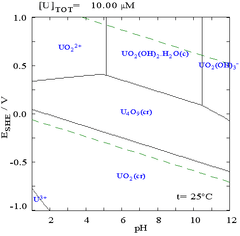
Pourbaix diagrams are also known as EH-pH diagrams due to the labeling of the two axes. The vertical axis is labeled EH for the voltage potential with respect to the standard hydrogen electrode (SHE) as calculated by the Nernst equation. The "H" stands for hydrogen, although other standards may be used, and they are for room temperature only.
The horizontal axis is labeled pH for the -log function of the H+ ion activity.
The lines in the Pourbaix diagram show the equilibrium conditions, that is, where the activities are equal, for the species on each side of that line. On either side of the line, one form of the species will instead be said to be predominant.[3]
In order to draw the position of the lines with the Nernst equation, the activity of the chemical species at equilibrium must be defined. Usually, the activity of a species is approximated as equal to the concentration (for soluble species) or partial pressure (for gases). The same values should be used for all species present in the system.[3]
For soluble species, the lines are often drawn for concentrations of 1 M or 10−6 M. Sometimes additional lines are drawn for other concentrations.
If the diagram involves the equilibrium between a dissolved species and a gas, the pressure is usually set to P0 = 1 atm = 101,325 Pa, the minimum pressure required for gas evolution from an aqueous solution at standard conditions.[3]
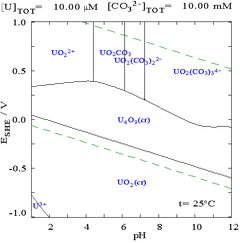
While such diagrams can be drawn for any chemical system, it is important to note that the addition of a metal binding agent (ligand) will often modify the diagram. For instance, carbonate has a great effect upon the diagram for uranium. (See diagrams at right.) The presence of trace amounts of certain species such as chloride ions can also greatly affect the stability of certain species by destroying passivating layers.
In addition, changes in temperature and concentration of solvated ions in solution will shift the equilibrium lines in accordance with the Nernst equation.
The diagrams also do not take kinetic effects into account, meaning that species shown as unstable might not react to any significant degree in practice.
A simplified Pourbaix diagram indicates regions of "Immunity", "Corrosion" and "Passivity", instead of the stable species. They thus give a guide to the stability of a particular metal in a specific environment. Immunity means that the metal is not attacked, while corrosion shows that general attack will occur. Passivation occurs when the metal forms a stable coating of an oxide or other salt on its surface, the best example being the relative stability of aluminium because of the alumina layer formed on its surface when exposed to air.
The stability region of water
In many cases, the possible conditions in a system are limited by the stability region of water. In the Pourbaix diagram for uranium, the limits of stability of water are marked by the two dashed green lines, and the stability region for water falls between these lines.
Under highly reducing conditions (low EH/pE) water will be reduced to hydrogen according to[3]
or
Using the Nernst equation, setting E0 = 0 V and the hydrogen gas fugacity (corresponding to activity) at 1, the equation for the lower stability line of water in the Pourbaix diagram will be
at standard temperature and pressure. Below this line, water will be reduced to hydrogen, and it will usually not be possible to pass beyond this line as long as there is still water present to be reduced.
Correspondingly, under highly oxidizing conditions (high EH/pE) water will be oxidized to oxygen gas according to[3]
Using the Nernst equation as above, but with an E0 of 1.229 V, gives an upper stability limit of water at
at standard temperature and pressure. Above this line, water will be oxidized to form oxygen gas, and it will usually not be possible to pass beyond this line as long as there is still water present to be oxidized.
Uses
Pourbaix diagrams have several uses, for example in corrosion studies, geoscience and in environmental studies.
In environmental chemistry
Pourbaix diagrams are widely used to describe the chemical behaviour of chemical species in the hydrosphere. In these cases, pE is used instead of EH.[3] pE is a dimensionless number and can easily be related to EH by the equation
pE values in environmental chemistry ranges from -12 to 25, since at low or high potentials water will be reduced or oxidized, respectively. In environmental applications, the concentration of dissolved species is usually set to a value between 10−2 M and 10−5 M for the creation of the equilibrium lines.
See also
References
- ↑ University of Bath & Western Oregon University
- 1 2 . Ignasi Puigdomenech, Hydra/Medusa Chemical Equilibrium Database and Plotting Software (2004) KTH Royal Institute of Technology, freely downloadable software at
- 1 2 3 4 5 6 vanLoon, Gary; Duffy, Stephen (2011). Environmental Chemistry - a global perspective (3rd ed.). Oxford University Press. pp. 235–248. ISBN 978-0-19-922886-7.
- Brookins, D. G., Eh-pH Diagrams for Geochemistry. 1988, Springer-Verlag, ISBN 0-387-18485-6
- Denny A. Jones, Principles and Prevention of Corrosion, 2nd edition, 1996, Prentice Hall, Upper Saddle River, NJ. ISBN 0-13-359993-0 Page 50-52
- Pourbaix, M., Atlas of electrochemical equilibria in aqueous solutions. 2d English ed. 1974, Houston, Tex.: National Association of Corrosion Engineers.
External links
| Wikimedia Commons has media related to Pourbaix diagrams. |
- Marcel Pourbaix — Corrosion Doctors
- DoITPoMS Teaching and Learning Package- "The Nernst Equation and Pourbaix Diagrams"
Software
- ChemEQL Free software for calculation of chemical equilibria from Eawag.
- FactSage Commercial thermodynamic databank software, also available in a free web application.
- The Geochemist's Workbench Commercial geochemical modeling software from Aqueous Solutions LLC.
- GWB Student Edition Free student edition of the popular geochemical modeling software package.
- HYDRA/MEDUSA Free software for creating chemical equilibrium diagrams from the KTH Department of Chemistry.
- HSC Chemistry Commercial thermochemical calculation software from Outotec Research Oy.
- PhreePlot Free program for making geochemical plots using the USGS code PHREEQC.
- Thermo-Calc Windows Commercial software for thermodynamic calculations from Thermo-Calc Software.
- Materials Project Public website that can generate Pourbaix diagrams from a large database of computed material properties, hosted at NERSC.