Phosphaalkene

Phosphaalkenes (IUPAC name: alkylidenephosphanes) are organophosphorus compounds with double bonds between carbon and phosphorus(III) with the formula R2C=PR. In the compound phosphorine one carbon atom in benzene is replaced by phosphorus. The reactivity of phosphaalkenes is often compared to that of alkenes and not to that of imines because the HOMO of phosphaalkenes is not the phosphorus lone pair (as in imines the amine lone pair) but the double bond. Therefore like alkenes, phosphaalkenes engage in Wittig reactions, Peterson reactions, Cope rearrangements, and Diels-Alder reactions.
The first phosphaalkene discovered was a phosphabenzene, by Mërkl in 1969. The first localized phosphaalkene was reported in 1974 by Gerd Becker[1] as a keto-enol tautomerism akin a Brook rearrangement:
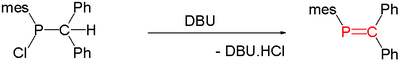
In the same year Harold Kroto established spectroscopically that thermolysis of Me2PH generates CH2=PMe. A general method for the synthesis of phosphaalkenes is by 1,2-elimination of suitable precursors, initiated thermally or by base such as DBU, DABCO or triethylamine:[2]
The Becker method is used in the synthesis of the phosphorus pendant of Poly(p-phenylene vinylene):[3]
.svg.png) .
.
References
- ↑ Bildung und Eigenschaften von Acylphosphinen. I. Monosubstitutionsreaktionen an substituierten Disilylphosphinen mit Pivaloylchlorid Gerd Becker, Zeitschrift für anorganische und allgemeine Chemie 1976, 423, pp 242 - 254
- ↑ Synthesis of mesityldiphenylmethylenephosphine: a stable compound with a localized phosphorus:carbon bond T. C. Klebach, R. Lourens, F. Bickelhaupt J. Am. Chem. Soc., 1978, 100 (15), pp 4886–4888 doi:10.1021/ja00483a041
- ↑ Phosphorus Copies of PPV: π-Conjugated Polymers and Molecules Composed of Alternating Phenylene and Phosphaalkene Moieties Vincent A. Wright, Brian O. Patrick, Celine Schneider, and Derek P. Gates J. Am. Chem. Soc.; 2006; 128(27) pp 8836 - 8844; (Article) doi:10.1021/ja060816l