Power to gas
Power to gas (also power-to-gas) (often abbreviated P2G) is a technology that converts electrical power to a gas fuel.[1] When using surplus power from wind generation, the concept is sometimes called windgas. There are currently three methods in use; all use electricity to split water into hydrogen and oxygen by means of electrolysis.
In the first method, the resulting hydrogen is injected into the natural gas grid or is used in transport or industry.[2] The second method is to combine the hydrogen with carbon dioxide and convert the two gases to methane (see natural gas) using a methanation reaction such as the Sabatier reaction, or biological methanation resulting in an extra energy conversion loss of 8%. The methane may then be fed into the natural gas grid. The third method uses the output gas of a wood gas generator or a biogas plant, after the biogas upgrader is mixed with the produced hydrogen from the electrolyzer, to upgrade the quality of the biogas.
Impurities, such as carbon dioxide, water, hydrogen sulfide, and particulates, must be removed from the biogas if the gas is used for pipeline storage to prevent damage.[3]
Storage function
Power-to-gas systems may be deployed as adjuncts to wind parks or solar-electric generation. The excess power or off-peak power generated by wind generators or solar arrays may then be used at a later time for load balancing in the energy grid. Before switching to natural gas, the German gas networks were operated using towngas, which for 50–60 % consisted of hydrogen. The storage capacity of the German natural gas network is more than 200,000 GWh which is enough for several months of energy requirement. By comparison, the capacity of all German pumped storage power plants amounts to only about 40 GWh. The storage requirement in Germany is estimated at 16GW in 2023, 80GW in 2033 and 130GW in 2050.[4] The transport of energy through a gas network is done with much less loss (<0.1%) than in a power network (8%). The storage costs per kilowatt hour are estimated at €0.10 for hydrogen and €0.15 for methane.[5] The use of the existing natural gas pipelines for hydrogen was studied by the EU NaturalHy project[6] and US DOE.[7] The blending technology is also used in HCNG.
Efficiency
In 2013 the round-trip efficiency of power-to-gas-storage was well below 50%, with the hydrogen path being able to reach an maximum efficiency of ~ 43% and methane of ~ 39% by using combined-cycle powerplants. If cogeneration plants are used that produce both electricity and heat, efficiency can be above 60%, but is still less than pumped hydro or battery storage.[8] However, there is potential to increase efficieny of power-to-gas storage. In 2015 a study published in Energy and Environmental Science found that by using reversible solid oxide electrochemical cells and recycling waste heat in the storage process a round-trip efficiency electricity to electricity of more than 70% can be reached at low cost.[9]
| Fuel | Efficiency | Conditions |
|---|---|---|
| Pathway: Electricity→Gas | ||
| Hydrogen | 54–72 % | 200 bar compression |
| Methane (SNG) | 49–64 % | |
| Hydrogen | 57–73 % | 80 bar compression (Natural gas pipeline) |
| Methane (SNG) | 50–64 % | |
| Hydrogen | 64–77 % | without compression |
| Methane (SNG) | 51–65 % | |
| Pathway: Electricity→Gas→Electricity | ||
| Hydrogen | 34–44 % | 80 bar compression up to 60% back to electricity |
| Methane (SNG) | 30–38 % | |
| Pathway: Electricity→Gas→Electricity & heat (cogeneration) | ||
| Hydrogen | 48–62 % | 80 bar compression and electricity/heat for 40/45 % |
| Methane (SNG) | 43–54 % | |
| Source: Fraunhofer IWES, p. 18, February 2011 (in German)[10] | ||
Electrolysis technology
- Advantages and disadvantages of the predominantly considered electrolysis technologies.[11]
| Advantage | Disadvantage |
|---|---|
| Commercial technology (high technology readiness level) | Limited cost reduction and efficiency improvement potential |
| Low investment electrolyser | High maintenance intensity |
| Large stack size | Modest reactivity, ramp rates and flexibility (minimal load 20%) |
| Extremely low hydrogen impurity (0,001 %) | Stacks < 250 kW require unusual AC/DC converters |
| Corrosive electrolyte deteriorates when not operating nominally |
| Advantage | Disadvantage |
|---|---|
| Reliable technology (no kinetics) and simple, compact design | High investment costs (noble metals, membrane) |
| Very fast response time | Limited lifetime of membranes |
| Cost reduction potential (modular design) | Requires high water purity |
| Advantage | Disadvantage |
|---|---|
| Highest electrolysis efficiency | Very low technology readiness level (proof of concept) |
| Low capital costs | Poor lifetime because of high temperature and affected material stability |
| Possibilities for integration with chemical methanation (heat recycling) | Limited flexibility; constant load required |
Power to hydrogen
In this method, electricity is used to split water into hydrogen and oxygen by means of electrolysis. The resulting hydrogen is injected into the natural gas grid or is used in transport or industry.[2]
ITM Power won a tender in March 2013 for a Thüga Group project, to supply a 360 kW self-pressurising high pressure electrolysis rapid response PEM electrolyser Rapid Response Electrolysis Power-to-Gas energy storage plant. The unit produces 125 kg/day of hydrogen gas and incorporates AEG power electronics. It will be situated at a Mainova AG site in the Schielestraße, Frankfurt in the state of Hessen. The operational data will be shared by the whole Thüga group – the largest network of energy companies in Germany with around 100 municipal utility members. The project partners include: badenova AG & Co. kg, Erdgas Mittelsachsen GmbH, Energieversorgung Mittelrhein GmbH, erdgas schwaben GmbH, Gasversorgung Westerwald GmbH, Mainova Aktiengesellschaft, Stadtwerke Ansbach GmbH, Stadtwerke Bad Hersfeld GmbH, Thüga Energienetze GmbH, WEMAG AG, e-rp GmbH, ESWE Versorgungs AG with Thüga Aktiengesellschaft as project coordinator. Scientific partners will participate in the operational phase.[12] It can produce 60 cubic metres of hydrogen per hour and feed 3,000 cubic metres of natural gas enriched with hydrogen into the grid per hour. An expansion of the pilot plant is planned from 2016, facilitating the full conversion of the hydrogen produced into methane to be directly injected into the natural gas grid.[13]

In December 2013, ITM Power, Mainova, and NRM Netzdienste Rhein-Main GmbH began injecting hydrogen into the German gas distribution network using ITM Power HGas, which is a rapid response proton exchange membrane electrolyser plant. The power consumption of the electrolyser is 315 kilowatts. It produces about 60 cubic meters per hour of hydrogen and thus in one hour can feed 3,000 cubic meters of hydrogen-enriched natural gas into the network.[14]
On August 28, 2013, E.ON Hanse, Solvicore, and Swissgas inaugurated a commercial power-to-gas unit in Falkenhagen, Germany. The unit, which has a capacity of two megawatts, can produce 360 cubic meters of hydrogen per hour.[15] The plant uses wind power and Hydrogenics[16] electrolysis equipment to transform water into hydrogen, which is then injected into the existing regional natural gas transmission system. Swissgas, which represents over 100 local natural gas utilities, is a partner in the project with a 20 percent capital stake and an agreement to purchase a portion of the gas produced. A second 800 kW power-to-gas project has been started in Hamburg/Reitbrook district[17] and is expected to open in 2015.[18]
In August 2013, a 140 MW wind park in Grapzow, Mecklenburg-Vorpommern ,owned by E.ON received an electrolyser. The hydrogen produced can be used in an internal combustion engine or can be injected into the local gas grid. The hydrogen compression and storage system stores up to 27 MWh of energy and increases the overall efficiency of the wind park by tapping into wind energy that otherwise would be wasted.[19] The electrolyser produces 210 Nm3/h of hydrogen and is operated by RH2-WKA.[20]
In January 2011, Secure Supplies Hydrogen, an internationally certified engineering and warrantied power to gas vendor of quality Australian and USA made Hydrogen Fueling equipment, Hydrogen Fueled Engines and Hydrogen Home /Business Solar fuel cell Kits entered the market. Project's deploying (2015-2020) are focused on renewable power investments. Value adding Sites to produce a Hydrogen fuel and transfer storage gas. Secure Supplies value adds all solar wind, geo thermal and hydropower projects. Enabling owners to achieve a higher ROI, to produce hydrogen gas grids to fuel industry and a variety of applications emission free. End users include Port Operators, Farms, Green Community residential development or and Renewable power operators. Gas is sold to gas grid or transported and used to fuel fixed engines or fuel cell that run 24r to pump water or make power for business and communities projects. Hydrogen Equipment along with Engineering and Service provision makes Secure Supplies is well positioned to supply key markets globally.[21]
The INGRID project started in 2013 in Apulia, Italy. It is a four-year project with 39 MWh storage and a 1.2 MW electrolyser for smart grid monitoring and control.[22] The hydrogen is used for grid balancing, transport, industry, and injection into the gas network.[23]
The surplus energy from the 12 MW Prenzlau Windpark in Brandenburg, Germany[24] will be injected into the gas grid from 2014 on.
The 6 MW Energiepark Mainz[25] from Stadtwerke Mainz, RheinMain University of Applied Sciences, Linde and Siemens in Mainz (Germany) will open in 2015.
Power to gas and other energy storage schemes to store and utilize renewable energy are part of Germany's Energiewende (energy transition program).[26]
Grid injection without compression
The core of the system is a proton exchange membrane (PEM) electrolyser. The electrolyser converts electrical energy into chemical energy, which in turn facilitates the storage of electricity. A gas mixing plant ensures that the proportion of hydrogen in the natural gas stream does not exceed two per cent by volume, the technically permissible maximum value when a natural gas filling station is situated in the local distribution network. The electrolyser supplies the hydrogen-methane mixture at the same pressure as the gas distribution network, namely 3.5 bar. [27]
Power to methane
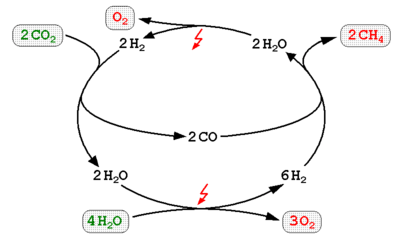
The Power to Gas Methane method is to combine hydrogen from an electrolyzer with carbon dioxide and convert the two gases to methane[28] (see natural gas) using a methanation reaction such as the Sabatier reaction or biological methanation resulting in an extra energy conversion loss of 8%, the methane may then be fed into the natural gas grid if the purity requirement is reached.
ZSW (Center for Solar Energy and Hydrogen Research) and SolarFuel GmbH (now ETOGAS GmbH) realized a demonstration project with 250 kW electrical input power in Stuttgart, Germany. The plant was put into operation on October 30, 2012.[29]
The first industry-scale Power-to-Methane plant was realized by ETOGAS for Audi AG in Werlte, Germany. The plant with 6 MW electrical input power is using CO2 from a waste-biogas plant and intermittent renewable power to produce synthetic natural gas (SNG) which is directly fed into the local gas grid (which is operated by EWE).[30] The plant is part of the Audi e-fuels program. The produced synthetic natural gas, named Audi e-gas, enables CO2-neutral mobility with standard CNG vehicles. Currently it is available to customers of Audi's first CNG car, the Audi A3 g-tron.[31]
In April 2014 the European Union’s co-financed and from the KIT coordinated[32] HELMETH[33] (Integrated High-Temperature ELectrolysis and METHanation for Effective Power to Gas Conversion) research project started.[34] The objective of the project is the proof of concept of a highly efficient Power-to-Gas technology by thermally integrating high temperature electrolysis (SOEC technology) with CO2-methanation. Through the thermal integration of exothermal methanation and steam generation for the high temperature steam electrolysis a conversion efficiency > 85% is expected (higher heating value of produced methane per used electrical energy). The process consists of a pressurized high-temperature steam electrolysis and a pressurized CO2-methanation module which are planned to be coupled in 2016. A methane output of approximately 30 kW (higher heating value) is targeted.
Microbial methanation
The biological methanation combines both processes, the electrolysis of water to form hydrogen and the subsequent CO2 reduction to methane using this hydrogen. During this process, methane forming microorganisms (methanogenic archaea or methanogens) release enzymes that reduce the overpotential of a non-catalytic electrode (the cathode) so that it can produce hydrogen.[35][36] This microbial power-to-gas reaction occurs at ambient conditions, i.e. room temperature and pH 7, at efficiencies that routinely reach 80-100%.[37][38] However, methane is formed more slowly than in the Sabatier reaction due to the lower temperatures. A direct conversion of CO2 to methane has also been postulated, circumventing the need for hydrogen production.[39] Microorganisms involved in the microbial power-to-gas reaction are typically members of the order Methanobacteriales. Genera that were shown to catalyze this reaction are Methanobacterium,[40][41] Methanobrevibacter,[42] and Methanothermobacter (thermophile).[43]
Biogas-upgrading to biomethane
In the third method the carbon dioxide in the output of a wood gas generator or a biogas plant after the biogas upgrader is mixed with the produced hydrogen from the electrolyzer to produce methane. The free heat coming from the electrolyzer is used to cut heating costs in the biogas plant. The impurities carbon dioxide, water, hydrogen sulfide, and particulates must be removed from the biogas if the gas is used for pipeline storage to prevent damage.
2014-Avedøre wastewater Services in Avedøre, Kopenhagen (Denmark) is adding a 1 MW electrolyzer plant to upgrade the anaerobic digestion biogas from sewage sludge.[44] The produced hydrogen is used with the carbon dioxide from the biogas in a Sabatier reaction to produce methane. Electrochaea[45] is testing another project outside P2G BioCat with biocatalytic methanation. The company uses an adapted strain of the thermophilic methanogen Methanothermobacter thermautotrophicus and has demonstrated its technology at laboratory-scale in an industrial environment.[46] A pre-commercial demonstration project with a 10,000-liter reactor vessel was executed between January and November 2013 in Foulum, Denmark.[47]
In 2016 Torrgas, Siemens, Stedin, Gasunie, A.Hak, Hanzehogeschool/EnTranCe and Energy Valley intend to open a 12 MW Power to Gas facility in Delfzijl (The Netherlands) where biogas from Torrgas (biocoal) will be upgraded with hydrogen from electrolysis and delivered to nearby industrial consumers.[48]
Power to syngas
- 1st step: Electrolysis of Water (SOEC) −water is split into hydrogen and oxygen.
- 2nd step: Conversion Reactor (RWGSR) −hydrogen and carbon dioxide are inputs to the Conversion Reactor that outputs hydrogen, carbon monoxide, and water.
3H2 + CO2 → (2H2 + CO)syngas + H2O
Initiatives
Other initiatives to create syngas from carbon dioxide and water may use different water splitting methods.
- CSP
- HTE / Alkaline water electrolysis
- 2004 Syntrolysis Fuels —Idaho National Laboratory and Ceramatec, Inc. (US).[65][66][67][68][69][70]
- 2008 WindFuels —Doty Energy (US).[71][72]
- 2012 Air Fuel Synthesis —Air Fuel Synthesis Ltd (UK).[73][74][75][76][77]
- 2013 Green Feed —BGU and Israel Strategic Alternative Energy Foundation (I-SAEF).[78][79][80][81]
- 2014 E-diesel —Sunfire, a clean technology company and Audi.[82][83][84]
The US Naval Research Laboratory (NRL) is designing a power-to-liquids system using the Fischer-Tropsch Process to create fuel on board a ship at sea,[85] with the base products carbon dioxide (CO2) and water (H2O) being derived from sea water via "An Electrochemical Module Configuration For The Continuous Acidification Of Alkaline Water Sources And Recovery Of CO2 With Continuous Hydrogen Gas Production".[86][87]
See also
- Carbon-neutral fuel
- Grid energy storage
- List of energy storage projects
- Particulates
- Power-to-X
- Timeline of hydrogen technologies
Notes
- ↑ DLR-Power to gas in transport-Status quo and perspectives for development
- 1 2 Eberle, Ulrich; Mueller, Bernd; von Helmolt, Rittmar. "Fuel cell electric vehicles and hydrogen infrastructure: status 2012". Energy & Environmental Science. Retrieved 2014-12-16.
- ↑ NREL 2013: Blending hydrogen into natural gas pipeline networks: A review of key issues
- ↑ Electricity storage in the German energy transition
- ↑ "Wind power to hydrogen". hi!tech. Siemens. Retrieved 2014-06-21.
- ↑ NaturalHY Project. "Using the Existing Natural Gas System for Hydrogen". EXERGIA. Retrieved 2014-06-21.
- ↑ NREL - Blending hydrogen into natural gas pipeline networks A review of key issues
- ↑ Volker Quaschning, Regenerative Energiesysteme. Technologie - Berechnung - Simulation, Hanser 2013, p 373.
- ↑ Jensen et al, Large-scale electricity storage utilizing reversible solid oxide cells combined with underground storage of CO2 and CH4. In: Energy and Environmental Science 8, (2015), 2471-2479, doi:10.1039/c5ee01485a.
- ↑ (German) Fraunhofer -Energiewirtschaftliche und ökologische Bewertung eines Windgas-Angebotes, p. 18
- ↑ Power-to-gas: Climbing the technology readiness ladder
- ↑ http://www.itm-power.com/news-item/first-sale-of-power-to-gas-plant-in-germany/
- ↑ Ground broken at ITM Power power-to-gas pilot plant in Frankfurt
- ↑ http://www.itm-power.com/news-item/injection-of-hydrogen-into-the-german-gas-distribution-grid/
- ↑ http://www.eon.com/en/media/news/press-releases/2013/8/28/eon-inaugurates-power-to-gas-unit-in-falkenhagen-in-eastern-germany.html
- ↑ Hydrogenics and Enbridge to develop utility-scale energy storage
- ↑ E.on Hanse starts construction of power-to-gas facility in Hamburg
- ↑ E.ON power-to-gas pilot unit in Falkenhagen first year of operation
- ↑ German wind park with 1 MW Hydrogenics electrolyser for power-to-gas energy storage
- ↑ RH2-WKA
- ↑
- ↑ INGRID Project to Launch 1.2 MW Electrolyser with 1 Ton of Storage for Smart Grid Balancing in Italy
- ↑ Grid balancing, Power to Gas (PtG)
- ↑ Prenzlau Windpark (Germany)
- ↑ Energiepark Mainz
- ↑ Schiermeier, Quirin (April 10, 2013). "Renewable power: Germany's energy gamble: An ambitious plan to slash greenhouse-gas emissions must clear some high technical and economic hurdles.". Nature. Retrieved April 10, 2013.
- ↑
- ↑ DNV-Kema Systems analyses power to gas
- ↑ http://www.zsw-bw.de/infoportal/presseinformationen/presse-detail/weltweit-groesste-power-to-gas-anlage-zur-methan-erzeugung-geht-in-betrieb.html
- ↑ http://www.audi.com/content/com/brand/en/vorsprung_durch_technik/content/2013/10/energy-turnaround-in-the-tank.html
- ↑ http://www.audi.com/corporate/en/corporate-responsibility/we-live-responsibility/product/audi-e-gas-new-fuel.html
- ↑ "Engler-Bunte-Institute Division of Combustion Technology - Project HELMETH". Retrieved 2014-10-31.
- ↑ "Project homepage - HELMETH". Retrieved 2014-10-31.
- ↑ "Karlsruhe Institute of Technology - Press Release 044/2014". Retrieved 2014-10-31.
- ↑ "Deutzmann, J. S.; Sahin, M.; Spormann, A. M., Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio 2015, 6, (2).".
- ↑ "Yates, M. D.; Siegert, M.; Logan, B. E., Hydrogen evolution catalyzed by viable and non-viable cells on biocathodes. Int. J. Hydrogen Energy 2014, 39, (30), 16841-16851.".
- ↑ "Marshall, C. W.; Ross, D. E.; Fichot, E. B.; Norman, R. S.; May, H. D., Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl. Environ. Microbiol. 2012, 78, (23), 8412-8420.".
- ↑ "Siegert, M.; Yates, M. D.; Call, D. F.; Zhu, X.; Spormann, A.; Logan, B. E., Comparison of nonprecious metal cathode materials for methane production by electromethanogenesis. ACS Sustainable Chemistry & Engineering 2014, 2, (4), 910-917.".
- ↑ "Cheng, S.; Xing, D.; Call, D. F.; Logan, B. E., Direct biological conversion of electric current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, (10), 3953-3958.".
- ↑ Pascal F. Beese-Vasbender, Jan-Philipp Grote, Julia Garrelfs, Martin Stratmann, Karl J.J. Mayrhofer: Selective microbial electrosynthesis of methane by a pure culture of a marine lithoautotrophic archaeon. In: Bioelectrochemistry. 102, 2015, S. 50, doi:10.1016/j.bioelechem.2014.11.004.
- ↑ Michael Siegert, Matthew D. Yates, Alfred M. Spormann, Bruce E. Logan: Methanobacterium dominates biocathodic archaeal communities in methanogenic microbial electrolysis cells. In: ACS Sustainable Chemistry & Engineering. 3(7), 2015, S. 1668, doi:10.1021/acssuschemeng.5b00367.
- ↑ Michael Siegert, Xiu-Fen Li, Matthew D. Yates, Bruce E. Logan: The presence of hydrogenotrophic methanogens in the inoculum improves methane gas production in microbial electrolysis cells. In: Frontiers in Microbiology. 5, 2015, doi:10.3389/fmicb.2014.00778.
- ↑ Kozo Sato, Hideo Kawaguchi, Hajime Kobayashi: Bio-electrochemical conversion of carbon dioxide to methane in geological storage reservoirs. In: Energy Conversion and Management. 66, 2013, S. 343, doi:10.1016/j.enconman.2012.12.008.
- ↑ Excess wind power is turned into green gas in Avedøre
- ↑ Electrochaea
- ↑ http://www.hindawi.com/journals/archaea/2013/157529/
- ↑ http://www.electrochaea.com/technology.html
- ↑ Power-to-Gas plant for Delfzijl
- ↑ "Sunshine to Petrol". Sandia National Laboratories. United States Department of Energy (DOE). Retrieved 15 May 2015.
- ↑ SNL: Sunshine to Petrol - Solar Recycling of Carbon Dioxide into Hydrocarbon Fuels
- ↑ "Sandia and Sunshine-to-Petrol™: Renewable Drop-in Transportation Fuels". Federal Business Opportunities. U.S. Federal Government. Oct 29, 2013. Retrieved 15 May 2015.
- ↑ Biello, David (September 23, 2010). "Reverse Combustion: Can CO2 Be Turned Back into Fuel?". Scientific American - Energy & Sustainability. Scientific American, a Division of Nature America, Inc. Retrieved 17 May 2015.
- ↑ Lavelle, Marianne (August 11, 2011). "Carbon Recycling: Mining the Air for Fuel". National Geographic - News. National Geographic Society. Retrieved 19 May 2015.
- ↑ Onur Taylan and Halil Berberoglu (2013). Fuel Production Using Concentrated Solar Energy, Application of Solar Energy, Prof. Radu Rugescu (Ed.), ISBN 978-953-51-0969-3, InTech, DOI: 10.5772/54057. Available from: Intechopen.com
- ↑ "Bright Way to Convert Greenhouse Gas to Biofuel". Weizmann UK. Weizmann UK. Registered Charity No. 232666. 18 December 2012. Retrieved 19 May 2015.
- ↑ "CO2 and H2O Dissociation Process". NCF - Technology Process. New CO2 Fuels Ltd. Retrieved 19 May 2015.
- ↑ Newsletter NewCO2Fuels, Issue 1, September 2012
- ↑ From challenge to opportunity New CO
2 Fuels: An Introduction... - ↑ "SOLAR-JET Project". SOLAR-JET. SOLAR-JET Project Office: ARTTIC. Retrieved 15 May 2015.
- ↑ "Sunlight to jet fuel". The ETH Zurich. Eidgenössische Technische Hochschule Zürich. Retrieved 15 May 2015.
- ↑ Alexander, Meg (May 1, 2014). ""Solar" jet fuel created from water and carbon dioxide". Gizmag. Gizmag. Retrieved 15 May 2015.
- ↑ "SOLARJET demonstrates full process for thermochemical production of renewable jet fuel from H2O & CO2". Green Car Congress. BioAge Group, LLC. 28 April 2015. Retrieved 15 May 2015.
- ↑ "Aldo Steinfeld - Solar Syngas". Solve For <X>. Google Inc.
- ↑ Brewing fuels in a solar furnace
- ↑ Syntrolysis, Synthetic Fuels from Carbon Dioxide, Electricity and Steam
- ↑ "Synthetic Fuel (syntrolysis)". Thoughtware.TV. Thoughtware.TV. June 17, 2008. Retrieved 20 May 2015.
- ↑ STOOTS C M; O’BRIEN J E; HARTVIGSEN J (January 1, 2007). "Carbon neutral production of syngas via high temperature electrolytic reduction of steam and CO
2". ASME 2007 International Mechanical Engineering Congress and Exposition. ASME. 15: Sustainable Products and Processes: 185–194. doi:10.1115/IMECE2007-43667.STOOTS C M, O’BRIEN J E, HARTVIGSEN J. Carbon neutral production of syngas via high temperature electrolytic reduction of steam and CO
External link in
2 [C] //2007 ASME International Mechanical Engineering Congress and Exposition, Seattle, Washington, USA, 2007: 185-194.|title=(help) - ↑ Nuclear Hydrogen Initiative Overview
- ↑ Nuclear Hydrogen Production Technology
- ↑ Electrolysis For Synthetic Fuel Production
- ↑ "The WindFuels™ Primer - Basic Explanation for the Non-scientist". Doty Energy. Doty Energy. Retrieved 16 May 2015.
- ↑ Securing Our Energy Future by Efficiently Recycling CO2 into Transportation Fuels
- ↑ "The AFS Process - turning air into a sustainable fuel". Air Fuel Synthesis - Technical Review. Air Fuel Synthesis Limited. Retrieved 19 May 2015.
- ↑ Case Study: AFS demonstrator unit
- ↑ "Cars Fueled by Air?". PlanetForward.org. Planet Forward. Retrieved 20 May 2015.
- ↑ Rapier, Robert (October 31, 2012). "Investors Beware of Fuel from Thin Air". Investing Daily. Investing Daily, a division of Capitol Information Group, Inc. Retrieved 17 May 2015.
- ↑ K.R. WILLIAMS AND N. VAN LOOKEREN CAMPAGNE, SYNTHETIC FUELS FROM ATMOSPHERIC CARBON DIOXIDE
- ↑ "BGU Researchers invent Green Alternative to Crude Oil". Ben-Gurion University of the Negev. Ben-Gurion University of the Negev. 13 November 2013. Retrieved 17 May 2015.
- ↑ "Recent Success Story: Converting carbon dioxide, a damaging greenhouse gas, into fuel that may be used for transportation". I-SAEF. Israel Strategic Alternative Energy Foundation. Retrieved 15 May 2015.
- ↑ "BGU Researchers Develop New Type of Crude Oil Using Carbon Dioxide and Hydrogen". American Associates (Ben-Gurion University of the Negev). American Associates (AABGU). Retrieved 15 May 2015.
- ↑ "BGU researchers developing more efficient process for hydrogenation of CO2 to synthetic crude". Green Car Congress. BioAge Group, LLC. 21 November 2013. Retrieved 15 May 2015.
- ↑ "Fuel of the future: Research facility in Dresden produces first batch of Audi e-diesel". Audi MediaServices - Press release. Ingolstadt/Berlin: AUDI AG. 2015-04-21. Retrieved 23 May 2015.
- ↑ Rapier, Robert. "Is Audi's Carbon-Neutral Diesel a Game-Changer?". Energy Trends Insider. Energy Trends Insider. Retrieved 15 May 2015.
- ↑ Novella, Steven (28 April 2015). "Apr 28 2015 Audi's E-Diesel". The NeuroLogicaBlog - Technology. Steven Novella, MD. Retrieved 24 May 2015.
- ↑ "How the United States Navy Plans to Turn Seawater into Jet Fuel". Alternative Energy. altenergy.org. Retrieved 8 May 2015.
- ↑ "Patent: US 20140238869 A1". Google Patents. Google Inc. Retrieved 8 May 2015.
- ↑ The total carbon content of the world's oceans is roughly 38,000 GtC. Over 95% of this carbon is in the form of dissolved bicarbonate ion (HCO3 −). (Cline 1992, The Economics of Global Warming; Institute for International Economics: Washington D.C.). The dissolved bicarbonate and carbonate of the ocean is essentially bound CO2 and the sum of these species along with gaseous CO2, shown in the following equation, represents the total carbon dioxide concentration [CO2]T, of the world's oceans. Σ[CO2]T=[CO2(g)]l+[HCO3 −]+[CO3 2−]
Further reading
- Manuel Götz, Jonathan Lefebvre, Friedemann Mörs, Amy McDaniel Koch, Frank Graf, Siegfried Bajohr, Rainer Reimert, Thomas Kolb. Renewable Power-to-Gas: A technological and economic review. In: Renewable Energy 85, (2016), 1371–1390, doi:10.1016/j.renene.2015.07.066.
- Méziane Boudellal. "Le Power-to-Gas, Stockage de l'électricité d'origine renouvelable". 192 pages. In French only. Editor: Dunod, June 2016.
External links
- Zentrum für Sonnenenergie-und Wasserstoff-Forschung (ZSW) Baden-Württemberg
- Smedley, Tim. Power-to-gas energy storage could help displace use of fossil fuels, The Guardian, July 4, 2014. Retrieved from theguardian.com, July 21, 2014.