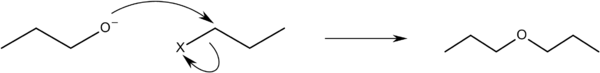Dipropyl ether
| | |
| Names | |
|---|---|
| IUPAC name
1-Propoxypropane | |
| Other names
propyl ether dipropyl ether di-n-propyl ether | |
| Identifiers | |
| 111-43-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 7823 |
| ECHA InfoCard | 100.003.518 |
| PubChem | 8114 |
| |
| |
| Properties | |
| C6H14O | |
| Molar mass | 102.18 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.75 g/cm3[1] |
| Melting point | −122 °C (−188 °F; 151 K)[1] |
| Boiling point | 90 °C (194 °F; 363 K)[1] |
| 3 g/L (20 °C) | |
| Hazards | |
| NFPA 704 | |
| Flash point | −18 °C (0 °F; 255 K)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dipropyl ether is the symmetrical ether of two n-propyl groups. It is a colorless, flammable liquid with a sweet odor typical of ethers.
Preparation
Acid catalyzed ether synthesis
Dipropyl ether can be synthesized by reacting two molecules of n-propanol in the presence of 4-methylbenezesulfonic acid (a strong acid) and heat, in the same way other symmetrical ethers may be formed.[2]
Williamson ether synthesis
This ether may also be prepared by way of the Williamson ether synthesis in which n-propoxide, the conjugate base of n-propanol, is reacted with an n-propyl halide:[3]
Safety
As is typical of ethers, dipropyl ether may slowly form explosive organic peroxides over long periods in storage.[2] Antioxidants such as butylated hydroxytoluene are often added to ethers to prevent this process.[4]
Due to the shock and light sensitive nature of organic peroxides, dipropyl ether should never be boiled or evaporated to dryness. This concentrates peroxides that may be present, which can then detonate unexpectedly destroying the vessel in which they have deposited or igniting nearby flammable liquids.[5]
See also
References
- 1 2 3 4 Record in the GESTIS Substance Database of the IFA
- 1 2 O'Neil, Maryadele; Heckelman, Patricia; Koch, Cherie; Roman, Kristin, eds. (2006). Merck Index of Chemicals and Drugs (14th ed.). Merck Research Laboratories. ISBN 978-0-911910-00-1.
- ↑ Fox, Marye; Whitesell, James (2004). Organic Chemistry, 3rd ed. Jones & Bartlett Publishers. ISBN 978-0763735869.
- ↑ "Diethyl ether product listing". Sigma-Aldrich. Retrieved 2012-07-03.
- ↑ "Organic peroxide hazards". Canadian Centre for Occupational Health and Safety. Retrieved 2012-07-03.