Tipifarnib
 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | R115777 |
| CAS Number |
192185-72-1 |
| PubChem (CID) | 159324 |
| IUPHAR/BPS | 8025 |
| DrugBank |
DB04960 |
| ChemSpider |
140122 |
| UNII |
MAT637500A |
| KEGG |
D03720 |
| ChEMBL |
CHEMBL289228 |
| Chemical and physical data | |
| Formula | C27H22Cl2N4O |
| Molar mass | 489.40 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Tipifarnib (INN,[1]:213 proposed trade name Zarnestra) is a farnesyltransferase inhibitor that is being investigated in patients 65 years of age and older with newly diagnosed acute myeloid leukemia (AML). It inhibits the Ras kinase in a post-translational modification step before the kinase pathway becomes hyperactive. It inhibits prenylation of the CxxX tail motif, which allows Ras to bind to the membrane where it is active. Without this step the protein cannot function.
It is also being tested in clinical trials in patients in certain stages of breast cancer.[2] It is also investigated as a treatment for multiple myeloma.[3]
For treatment of progressive plexiform neurofibromas associated with neurofibromatosis type I, it successfully passed phase I clinical trials but was suspended (NCT00029354) in phase II.[4][5] The compound was discovered by and is under investigation by Johnson & Johnson Pharmaceutical Research & Development, L.L.C, with registration number R115777.
Approval process
Tipifarnib was submitted to the FDA by Johnson & Johnson for the treatment of AML in patients aged 65 and over with a new drug application (NDA) to the FDA on January 24, 2005.
In June 2005, the FDA issued a "not approvable" letter for tipifarnib.[6]
Progeria
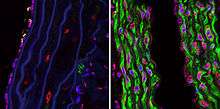
It was shown on a mouse model of Hutchinson–Gilford progeria syndrome that dose-dependent administration of tipifarnib can significantly prevent both the onset of the cardiovascular phenotype as well as the late progression of existing cardiovascular disease.[7]
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names (Rec. INN): List 46" (PDF). World Health Organization. Retrieved 16 November 2016.
- ↑ Sparano, JA; Moulder, S; Kazi, A; Coppola, D; Negassa, A; Vahdat, L; Li, T; Pellegrino, C; Fineberg, S; Munster, P; Malafa, M; Lee, D; Hoschander, S; Hopkins, U; Hershman, D; Wright, JJ; Kleer, C; Merajver, S; Sebti, SM (15 April 2009). "Phase II Trial of Tipifarnib plus Neoadjuvant Doxorubicin-Cyclophosphamide in Patients with Clinical Stage IIB-IIIC Breast Cancer" (PDF). Clinical Cancer Research. 15 (8): 2942–48. doi:10.1158/1078-0432.CCR-08-2658. PMC 2785076
 . PMID 19351752. Retrieved 16 November 2016.
. PMID 19351752. Retrieved 16 November 2016. - ↑ Alsina, M; Fonseca, R; Wilson, EF; Belle, AN; Gerbino, E; Price-Troska, T; Overton, RM; Ahmann, G; Bruzek, LM; Adjei, AA; Kaufmann, SH; Wright, JJ; Sullivan, D; Djulbegovic, B; Cantor, AB; Greipp, RP; Dalton, WS; Sebti, SM (1 May 2004). "Farnesyltransferase Inhibitor Tipifarnib Is Well Tolerated, Induces Stabilization of Disease, and Inhibits Farnesylation and Oncogenic/Tumor Survival Pathways in Patients with Advanced Multiple Myeloma" (PDF). Blood. 103 (9): 3271–7. doi:10.1182/blood-2003-08-2764. PMID 14726402. Retrieved 16 November 2016.
- ↑ "R115777 in Treating Patients With Advanced Solid Tumors"
- ↑ "R115777 to Treat Children With Neurofibromatosis Type 1 and Progressive Plexiform Neurofibromas"
- ↑ "Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Receives Not Approvable Letter From FDA for Tipifarnib Based on Phase II Data". PR Newswire. Jun 30, 2005. Retrieved 16 November 2016.
- ↑ Capell, BC; Olive, M; Erdos, MR; Cao, K; Faddah, DA; Tavarez, UL; Conneely, KN; Qu, X; San, H; Ganesh, SK; Chen, X; Avallone, H; Kolodgie, FD; Virmani, R; Nabel, EG; Collins, FS (6 October 2008). "A Farnesyltransferase Inhibitor Prevents Both the Onset and Late Progression of Cardiovascular Disease in a Progeria Mouse Model" (PDF). Proceedings of the National Academy of Sciences. 105 (41): 15902–7. doi:10.1073/pnas.0807840105. PMC 2562418
 . PMID 18838683. Retrieved 16 November 2016.
. PMID 18838683. Retrieved 16 November 2016.