Valrubicin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611021 |
| Pregnancy category |
|
| Routes of administration | Intravesical |
| ATC code | L01DB09 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Negligible |
| Protein binding | >99% |
| Metabolism | Negligible |
| Excretion | In urine |
| Identifiers | |
| |
| CAS Number |
56124-62-0 |
| PubChem (CID) | 454216 |
| DrugBank |
DB00385 |
| ChemSpider |
399974 |
| UNII |
2C6NUM6878 |
| ChEMBL |
CHEMBL1096885 |
| ECHA InfoCard | 100.205.793 |
| Chemical and physical data | |
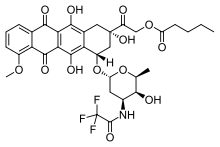
| Formula | C34H36F3NO13 |
| Molar mass | 723.644 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Valrubicin (N-trifluoroacetyladriamycin-14-valerate, trade name Valstar) is a chemotherapy drug used to treat bladder cancer. Valrubicin is a semisynthetic analog of the anthracycline doxorubicin, and is administered by infusion directly into the bladder.
It was originally launched as Valstar in the U.S. in 1999 for intravesical therapy of Bacille Calmette-Guérin (BCG)-refractory carcinoma in situ of the urinary bladder in patients in whom cystectomy would be associated with unacceptable morbidity or mortality; however, it was voluntarily withdrawn in 2002 due to manufacturing issues.[1] Valstar was relaunched on September 3, 2009.[2]
Side effects
- Blood in urine
- Incontinence
- painful or difficult urination
- Unusually frequent urination
References
- ↑ "Manufacturing Issues Remain for Indevus' Valstar", U.S. Food and Drug Administration News. The MQN Weekly Bulletin, Jan. 11, 2008
- ↑ "Endo Pharmaceuticals launches VALSTAR for treating recurrent carcinoma in situ bladder tumors" (Press release). 2009-09-03. Retrieved 2009-11-26.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.