Ractopamine
 | |
| Names | |
|---|---|
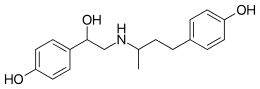
| IUPAC name
4-[3-[[2-Hydroxy-2-(4-hydroxyphenyl)ethyl]amino]butyl]phenol | |
| Identifiers | |
| 97825-25-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:82644 |
| ChEMBL | ChEMBL509336 |
| ChemSpider | 50604 |
| MeSH | Ractopamine |
| PubChem | 56052 |
| UNII | 57370OZ3P1 |
| |
| |
| Properties | |
| C18H23NO3 | |
| Molar mass | 301.39 g·mol−1 |
| 4100 mg/L | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ractopamine is a feed additive to promote leanness in animals raised for their meat. Pharmacologically, it is a TAAR1 agonist and β adrenoreceptor agonist that stimulates β1 and β2 adrenergic receptors.[1][2] It is the active ingredient in products known as Paylean for swine and Optaflexx for cattle, developed by Elanco Animal Health, a division of Eli Lilly and Company, for use in food animals for growth promotion.
Ractopamine use has been banned in most countries, including the European Union, mainland China and Russia[3][4] while 27 other countries, such as Japan, the United States, Canada, and South Korea, have deemed meat from livestock fed ractopamine safe for human consumption.[5]
Commercial ractopamine is a mixture of all four possible stereoisomers.[6]
Mode of action
When used as a food additive, ractopamine added to feed can be distributed via the blood to the muscle tissues, where it serves as a full agonist at mouse (not necessarily human) TAAR1.[1] It is also an agonist at beta-adrenergic receptors.[2] A cascade of events will then be initiated to increase protein synthesis, which results in increased muscle fiber size. Ractopamine is known to increase the rate of weight gain, improve feed efficiency, and increase carcass leanness in finishing swine. Its use in finishing swine yields about three kilograms of additional lean pork and improves feed efficiency by 10%.[7]
Regulation around the world
As of 2015, ractopamine use as a feed additive is authorized in the United States, Canada, and Mexico.[8] The American Institute in Taiwan, which represents U.S. interests in Taiwan, claims that these "and many other countries have determined that meat from animals fed ractopamine is safe for human consumption";[5] this is in the context of an ongoing trade dispute between Taiwan and the U.S. on this subject, which threatened to prevent Taiwan's entry to the Trans-Pacific Partnership trade pact.[9] In the U.S., ractopamine is allowed to be used at a feed concentration of 5–20 mg/kg feed for finishing pigs and in dosages of 5–10 mg/kg feed for finishing pigs heavier than 109 kg. The maximum residue limit for ractopamine for meat in the USA is 50 ppb.
In Canada, ractopamine is only allowed in meal or pellet feed for finishing barrows and gilts, confined finishing cattle, and finishing heavy turkeys.[10]
Japan, which had permitted its feed additive use at least until 2009,[8]:1 and South Korea only allow import of meat with ractopamine residues up to the maximum residue limit (MRL), but do not permit its use in beef production.[11]
On 6 July 2012, the international reference standard Codex Alimentarius Commission narrowly approved the adoption of a maximum residue limit (MRL) of 10 parts per billion (ppb) for muscle cuts of beef and pork.[12] Setting any limit was a controversial move. Countries with major meat export markets had been lobbying for the establishment of such a standard for several years in order to use it as leverage to erode individual national-level bans in World Trade Organization disputes.[12] Consumers International, a world federation of consumer groups that represents 220 consumer organizations in 115 countries, strongly opposed the move.[12]
As of 2013 Ractopamine use in food animals has been banned in over 160 countries.[13] It has not been allowed in the 27 member countries of the European Union, based on the 2009 European Food Safety Authority's (EFSA) opinion on its safety evaluation, which concluded that available data were insufficient to derive a maximum residue limit (MRL) as a 'safe residue level for human consumption'. The uncertainty was particularly great for people who might be thought to be more susceptible than most to an increase in β adrenergic stimulation from consuming the additive, such as people with cardiovascular disease or children, and that simply increasing the "uncertainty factor" built into the calculation as a safety factor would rapidly become arbitrary.[8][14]
Russia and China banned ractopamine in pork,[15] and Russia also in beef,[13] deeming it unfit for human consumption. Taiwan banned ractopamine along with other beta-adrenergic agonists in October 2006,[16] but in 2012 its legislature passed amendments to the Act Governing Food Sanitation, authorising government agencies to set safety standards for ractopamine.[17] The Department of Health ultimately established a maximum residual level of 10 ppb for ractopamine in beef on 31 July 2012.[18]
Pharmacokinetics in humans
A study was conducted to define the pharmacological response of humans to ractopamine. A single oral dose of 40 mg of ractopamine hydrochloride was given to human volunteers. The drug was rapidly absorbed; the mean blood plasma half-life was around 4 hrs and it was not detected in plasma 24 hrs after dosing. Less than 5% of total ractopamine excreted represented the parent drug, while the urinary metabolites were monoglucuronide and monosulfate conjugates, with ractopamine monosulfate being the major metabolite present.[19]
The metabolic fate of ractopamine hydrochloride is similar in the target species (pigs and cattle), laboratory animals, and humans. Besides the pharmacology effect, ractopamine may cause intoxication effect; therefore, any consumption by humans of a meat and/or byproducts of animals that consumed ractopamine with feed for growth stimulation, may result in such clinical effects as tachycardia and other heart rate increases, tremor, headache, muscle spasm, or high arterial blood pressure.[20] The effect of ractopamine on humans is not entirely known, but consumption of products that contain ractopamine residues is not advisable to people with cardiovascular diseases.
Safety concerns
Target animal safety
Ractopamine is safe for finishing pigs heavier than 240 lb (110 kg) when administered in the diet at concentrations up to 10 ppm and fed for up to 35 days. However, the number of ractopamine hydrochloride–treated animals exhibiting signs of injury increased during the final drive to slaughter. Since the drug was introduced, more than 160,000 pigs taking ractopamine were reported to have suffered adverse effects, as of March 2011, according to a review of FDA records. The drug has triggered more adverse reports in pigs than any other animal drug on the market. Pigs suffered from hyperactivity, trembling, broken limbs, inability to walk and death, according to FDA reports released under a Freedom of Information Act request.(FDA)[21]
Colorado State University Professor of Animal Science Temple Grandin, an expert on animal welfare, says that she has personally seen harmful effects of ractopamine on feedlot animals, such as cattle with stiff, sore, and lame limbs and symptoms of heat stress, and has stated that some cattle have lost feet due to extreme ractopamine overdoses.[22] She also asserts that meat from ractopamine-treated animals is tougher.[22]
Some show lambs have been illegally given ractopamine, either intentionally or due to accidental contamination of their feed.[23]
Adverse effects
Acute toxicity
Oral LD50 levels in mice and rats are 3547–2545 mg/kg body weight (male and female) and 474–365 (male and female), respectively.[24]
Genotoxicity and mutagenicity
Mutation studies in prokaryotes and eukaryotes show that ractopamine is not mutagenic. However, the results of several in vitro studies, including chromosome aberration tests in human lymphocytes, are positive. The positive genotoxic results are explained with limited evidence to be due to a secondary auto-oxidative mechanism from ractopamine-catechol-producing reactive intermediates.
Carcinogenicity
Ractopamine is not considered to be a carcinogen and not listed by IARC, NTP, ACGIH, or OSHA. The observation benign leiomyomas (tumors of smooth muscle) in mice and rats could be due to a general feature of beta-adrenergic activity of ractopamine.
Cardiovascular effects
Dose-dependent changes of heart rate and cardiac output are observed within the first hour after administration of ractopamine and gradually return to baseline values. The systolic blood pressure will also increase in a dose-dependent manner, while the diastolic pressure remains unchanged.
Musculoskeletal effects
Skeletal muscle tremor is the most common adverse effect of beta-agonists, and is more likely to be seen after oral administration than after inhalation. Tremor results from an imbalance between fast- and slow-twitch muscle groups of the extremities, and its severity varies greatly between individuals. No such effects were recorded at the NOEL determined in the toxicological studies conducted in laboratory animals given ractopamine or in the study in humans on cardiovascular effects of ractopamine.
Behavioral changes in humans
Restlessness, apprehension, and anxiety were reported effects after the use of various beta-agonists, particularly after oral or parenteral treatment. In pilot clinical trials with ractopamine, four patients showed little evidence for central nervous system stimulation. It is unclear whether long-term treatment with these drugs results in the development of tolerance to these adverse effects.
Analytical method for residues in meat
The determinative procedure for the analysis of ractopamine residues in tissue can be performed, using liver or muscle as the target tissues, by high performance liquid chromatography with fluorescence detection. The confirmatory method include reversed-phase HPLC/electrospray ionization triple tandem quadrupole mass spectrometry. The limit of quantification of the drug using this LC/MS instrument was shown to be 1 ng/g.[25]
International controversies
China
In July 2007, officials of the People's Republic of China seized US-produced pork for containing ractopamine residues.[26] Further shipments of ractopamine-fed pork were seized in September, though this time they were Canadian in origin.[27]
Taiwan
Ractopamine has been banned in Taiwan since 2006.[28] In the summer of 2007, two US shipments including ractopamine-laced pork were rejected by Taiwan's health authorities, while the Taiwan government had been considering lifting the ban on such imports.[29] This resulted in mass protests in the capital city, Taipei, by swine farmers insisting that the ban remain in place. Health Minister Hou Sheng-mou (侯勝茂) declared there would be no lifting of the ban unless related laws were amended. In August 2012, Taiwan's legislature passed amendments to the food safety act allowing ractopamine in beef.
Malaysia
According to the Malaysian Food Act 1983 and Regulations (as of 5 January 2010), ractopamine is allowed in pig muscle and fat (MRL of 10 ppb), pig liver (MRL of 40 ppb) and pig kidney (MRL of 90 ppb).[30] Ractopamine is allowed as its half-life is lower, leading to reduced residues in the food, and the dose required to affect humans is much higher than other beta agonists.[31] On 30 December 2008, the Malaysian Veterinary Services Department quarantined 10 of the 656 pig farms in Malaysia, as the livestock were found to contain the banned chemical.[32][33]
Russia
The use of ractopamine in Russia is prohibited. On 6 June 2011, the Russian Ministry of Agriculture notified key meat import/exporters in Russia of a future prohibition of ractopamine in meat imported to Russia.
On 7 December 2012, the prohibition went into force, and pork and beef export to Russia required submission of compliance certificates confirming absence of ractopamine in exported meat.
Comparison to clenbuterol
Similar to ractopamine, clenbuterol is a growth-promoting compound belonging to the β agonist family. It is known to have the effect of enhancing weight gain and proportion of muscle to fat. However, clenbuterol is known to have a much longer half-life in blood than ractopamine, thus has a greater potential for bioaccumulation .
Clenbuterol is reported to induce unintended side effects on humans, such as increased heart rate, muscular tremors, headache, nausea, fever, and chills. The US FDA has concluded these side effects to be unacceptable . The use of clenbuterol in food animals has been prohibited in almost all countries .
See also
References
- 1 2 Liu, X; Grandy, DK; Janowsky, A (July 2014). "Ractopamine, a livestock feed additive, is a full agonist at trace amine-associated receptor 1.". The Journal of Pharmacology and Experimental Therapeutics. 350 (1): 124–9. doi:10.1124/jpet.114.213116. PMID 24799633.
- 1 2 Colbert, WE; Williams, PD; Williams, GD (December 1991). "Beta-adrenoceptor Profile of Ractopamine HCl in Isolated Smooth and Cardiac Muscle Tissues of Rat and Guinea-pig". The Journal of Pharmacy and Pharmacology. 43 (12): 844–7. doi:10.1111/j.2042-7158.1991.tb03192.x. PMID 1687583.
- ↑ Bottemiller, Helena (January 25, 2012). "Dispute over drug in feed limiting US meat exports". Bottom Line.
- ↑ Garina, Anastasia. "Russia throws poisonous meat back to US". Pravda.ru. Retrieved 14 December 2012.
- 1 2 "The Facts about U.S. Beef and Ractopamine". American Institute in Taiwan. Retrieved 5 March 2012.
- ↑ Vivian Vezzoni de AlmeidaI; Amoracyr José Costa NuñezII; Valdomiro Shigueru Miyada (May 2012). "Ractopamine as a metabolic modifier feed additive for finishing pigs: a review" (PDF). Brazilian Archives of Biology and Technology. 25 (3). Retrieved 9 December 2014.
- ↑ Apple, JK; Rincker, PJ; McKeith, FK; Carr, SN; Armstrong, TA; Matzat, PAS; Matzat, PD (June 2007). "Meta-analysis of the ractopamine response in finishing swine" (PDF). The Professional Animal Science. 23: 179–196.
- 1 2 3 http://www.globalmeatnews.com/Industry-Markets/EU-opposes-ractopamine-limit
- ↑ Lin, Enru (December 30, 2015). "Taiwan's pig farmers threaten large-scale protest over US pork". China Post. Asia News Network. Retrieved 15 January 2016.
- ↑ Canadian Food Inspection Agency (September 2014). "Ractopamine Hydrochloride - MIB #82". Retrieved 29 November 2014.
- ↑ "Description of the Ractopamine Standards in Livestock Products of Different Countries". US Food and Drug Administration, Ministry of Health and Welfare. 23 March 2012. Retrieved 27 November 2014.
- 1 2 3 Bottemiller, Helena (July 6, 2012). "Codex Adopts Ractopamine Limits for Beef and Pork". Food Safety News. Retrieved 29 November 2014.
- 1 2 Russian Ban On Ractopamine Boosts Brazilian Exports Simon Quilty, Beefmagazine (Penton), March 27, 2013
- ↑ Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed(FEEDAP) on a request from the European Commission on the safety evaluation of ractopamine The EFSA Journal (2009) 1041, 1-52
- ↑ Smithfield Foods' profit falls 63% Michael Felberbaum, USA Today (AP) June 14, 2013
- ↑ "行政院農業委員會公告 (Council of Agriculture, Executive Yuan notice)" (in Chinese). 11 October 2006. Retrieved 28 June 2012.
- ↑ "Legislature votes to allow ractopamine". Taipei Times. 26 July 2012. Retrieved 3 August 2012.
- ↑ "DOH experts choose ractopamine residue cap for beef imports". Taiepei Times. CNA. 2 August 2012. Retrieved 3 August 2012.
- ↑ RACTOPAMINE (addendum). WHO FOOD ADDITIVES SERIES: 53
- ↑ "Safety evaluation of ractopamine EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP)". EFSA Journal. 7 April 2009. doi:10.2903/j.efsa.2009.1041. Retrieved 2013-03-21.
- ↑ "Dispute Over Drug in Feed Limiting US Meat Exports". The Fern. January 25, 2012.
- 1 2 Rockett, Caitlin (November 20, 2014). "Oops, we forgot to study that: U.S. nonprofits file suit against Food and Drug Administration over ractopamine in our meat". Boulder Weekly. Retrieved 29 November 2014.
- ↑ BERGIN, NICHOLAS (March 15, 2014). "Family sues feed company over lamb's failed drug test". Lincoln Journal Star.
- ↑ Safety evaluation of ractopamine (PDF). 2009. p. 10.
- ↑ Sakai T, et al. (2007). "Determination method for ractopamine in swine and cattle tissues using LC/MS". Shokuhin Eiseigaku Zasshi. 48 (5): 144–147. doi:10.3358/shokueishi.48.144. PMID 18027547.
- ↑ "China fights back, goes after U.S. meat", USA Today, July 14, 2007.
- ↑ "China stops imports from Canadian pork plant over banned additive", Canada Press (2007-09-19)
- ↑ Lin, Hermia (August 22, 2007). "Swine farmers get rowdy over ractopamine issue". Taiwan News. Archived from the original on September 29, 2011. Retrieved January 25, 2012.
- ↑ "Taiwanese farmers urge continuation of US pork import ban". Associated Press. August 21, 2007. Archived from the original on January 25, 2012.
- ↑ Fifteenth A Schedule, Table 1, Maximum Permitted Proportion of Drug Residues in Food.
- ↑ "Beta-agonists hog the limelight". The Star. November 5, 2006. Retrieved January 25, 2012.
- ↑ "Vet Dept seals 10 pig farms". The Star. December 31, 2008. Retrieved January 25, 2012.
- ↑ "10 pig farms under quarantine". The Star. December 30, 2008. Archived from the original on January 25, 2012.
External links
| Look up ractopamine in Wiktionary, the free dictionary. |