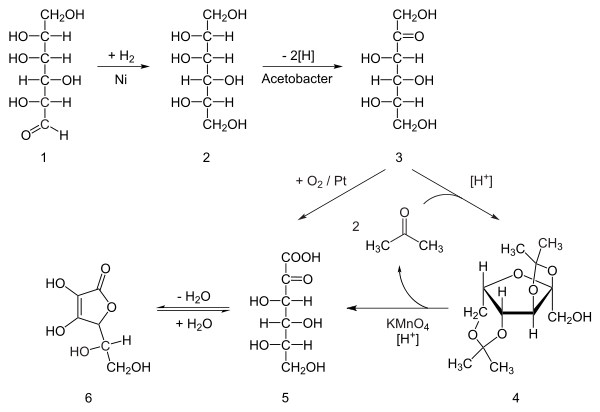
Reichstein process
The Reichstein process in chemistry is a combined chemical and microbial method for the production of ascorbic acid from D-glucose that takes place in several steps. This process was devised by Nobel Prize winner Tadeus Reichstein and his colleagues in 1933 while working in the laboratory of the ETH in Zürich.
Reaction steps
The reaction steps are:
- hydrogenation of D-glucose to D-sorbitol, an organic reaction with nickel as a catalyst under high temperature and high pressure.
- Microbial oxidation or fermentation of sorbitol to L-sorbose with acetobacter[1] at pH 4-6 and 30 °C.
- protection of the 4 hydroxyl groups in sorbose by formation of the acetal with acetone and an acid to Diacetone-L-sorbose (2,3:4,6−Diisopropyliden−α−L−sorbose)
- Organic oxidation with potassium permanganate followed by heating with water gives the 2-Keto-L-gulonic acid
- The final step is a ring-closing step or gamma lactonization with removal of water.[2]
- Intermediate 5 can also be prepared directly from 3 with oxygen and platinum
The microbial oxidation of sorbitol to sorbose is important because it provides the correct stereochemistry.
Importance
This process was patented and sold to Hoffmann-La Roche in 1935. The first commercially sold vitamin C product was called Cebion from Merck.
Even today all industrial methods for the production of ascorbic acid are based on the Reichstein process. In modern methods however, sorbose is directly oxidized with a platinum catalyst (developed by Kurt Heyns (1908–2005) in 1942). This method avoids the use of protective groups. A side product with particular modification is 5-Keto-D-gluconic acid.[3]
Novel methods involve genetically modified bacteria.[4]
References
- ↑ Wittko Francke und Wolfgang Walter: Lehrbuch der Organischen Chemie. S. Hirzel Verlag Stuttgart; 24. überarb Auflage 2004, ISBN 3-7776-1221-9; S. 480
- ↑ Reichstein, T. und Grüssner, A. (1934): Eine ergiebige Synthese der L-Ascorbinsäure (C-Vitamin), Helv. Chim. Acta 17, S. 311–328
- ↑ Brönnimann, C. et al. (1994): Direct oxidation of L-sorbose to 2-Keto-L-gulonic acid with molecular oxygen on Platinum- and Palladium-based catalysts. In: J. Catal. 150(1), S. 199–211; doi:10.1006/jcat.1994.1336
- ↑ Hancock, RD. und Viola, R. (2002): Biotechnological approaches for L-ascorbic acid production. In: Trends in Biotechnology 20(7); S. 299–305; PMID 12062975;doi:10.1016/S0167-7799(02)01991-1
Literature
- Boudrant, J. (1990): Microbial processes for ascorbic acid biosynthesis: a review. In: Enzyme Microb Technol. 12(5); 322–9; PMID 1366548; doi:10.1016/0141-0229(90)90159-N
- Bremus, C. et al. (2006): The use of microorganisms in L-ascorbic acid production. In: J Biotechnol. 124(1); 196–205; PMID 16516325; doi:10.1016/j.jbiotec.2006.01.010
External links
- http://www.chemieunterricht.de/dc2/asch2/a-synthe.htm
- http://www.tg.ethz.ch/forschung/projektbeschreib/Baechi/vitamin_c_synthese.htm