Retained blood syndrome
Retained blood syndrome (RBS) results from the ineffective evacuation of blood and fluid from a surgical or traumatic wound after surgery. This can happen after nearly any operation or major trauma, but especially operations like heart surgery, thoracic surgery, trauma surgery, general surgery, orthopedic surgery, neurosurgery, pelvic surgery or plastic surgery. In nearly all of these specialties, surgical drains are needed to remove blood and fluid from the surgical wounds in the early phase of recovery. RBS can develop when these drains fail to perform the required task that is to drain blood, fluid, and air from the surgical wound.
Although RBS can occur after any procedure that requires a drainage catheter, RBS is probably best recognized after cardiac surgery, where chest tubes are used to drain blood from around the heart and lungs in the early hours after surgery. If the evacuation of blood is incomplete, RBS can occur. Clinically, RBS can be recognized acutely or subacutely. When it presents acutely, it is usually fresh thrombus around the heart or lungs presenting as tamponade or hemothorax. When it presents subacutely, it results in bloody pleural or pericardial effusions.[1][2][3][4][5][6]
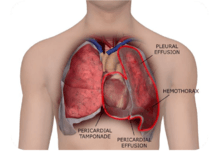
Once RBS occurs, subsequent procedures may be needed to remedy it. In an analysis of the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (NIS) data from 2010, RBS could be demonstrated in 17% of patients. In this analysis, mortality was doubled from 3% to 6%, length of stay was increased by 5 days, and average costs were 55% higher.[7] Patients with RBS, therefore, appear to be at increased at risk for complications and costs.
Recently it has become better understood that the postoperative obstruction of conventional chest tubes with blood and other fibrinous material in the setting of postoperative bleeding is central to the root cause of RBS.[8] When blood encounters the surfaces of chest tubes, the coagulation cascade is initiated, which often lead to partial or complete chest tube clogging with a blood clot. In a recent study of postoperative cardiac surgery patients at the Cleveland Clinic, 36% of patients were found to have evidence of chest tube obstruction.[2][9] If chest tube obstruction occurs when a patient is still bleeding after heart surgery, RBS ensues.
In a large study by Balzer and colleagues of 6,909 adult patients who underwent cardiac surgery, 1316 (19%) presented with a retained blood-related condition. Retained blood was associated with increased in-hospital mortality (odds ratio [OR], 4.041; 95% confidence interval [CI], 2.589-6.351, P < .001) and a length of stay more than 13 days in the hospital (OR, 3.853; 95% CI, 2.882-5.206; P < .001) and 5 days in the intensive care unit (OR, 4.602; 95% CI, 3.449-6.183; P < .001). The OR for a time of ventilation greater than 23 hours was 3.596 (95% CI, 2.690-4.851; P < .001) and for incidence of renal replacement therapy was 4.449 (95% CI, 3.188-6.226; P < .001).[10]
A growing body of literature suggests links between retained blood and the development of postoperative atrial fibrillation (POAF) and other complications.[11] Postoperative atrial fibrillation is one of the most common complications after heart surgery, occurring in 20% to 60% of patients, and is a leading cause of hospital readmissions.[12][13] Patients with occluded chest tubes have been shown to have a higher rate of POAF.[14] Although the precise mechanism that explains why patients with RBS have higher POAF is unclear, inflammation from clot in the pericardium may be contributory.[11] Retained blood syndrome may also contribute to the development of hospital acquired infections (HAIs). This may occur because the blood serves as a culture medium for bacteria. Or this may occur because patients with RBS are less mobile after surgery, a set up for infection. Finally, it could occur because these patients require more invasive interventions, which increase the risk of infection. Likely its a combination of all that explain why patients with RBS have an increase risk of hospital acquired infection.
Active clearance of chest tubes with the PleuraFlow Active Clearance Technology System can reduce RBS. In one study, a multidisciplinary team developed a simple protocol to institute active clearance to preventatively clear chest tubes of clot during the first 24 hours after heart surgery. In matched patients, 19.9% of patients had interventions for RBS (baseline). After the implementation of active clearance with PleuraFlow (treatment group), the percent of patients with interventions for RBS was reduced to 11.3%, representing a 43% reduction in RBS (P = .0087). These patients had a 33% reduced incidence of postoperative atrial fibrillation (POAF) with PleuraFlow.[15] These findings underscore the importance of maintaining chest tube patency in the early hours after cardiac surgery.
The costs of RBS are high. In one analysis of over 313,000 adult cardiac surgery patients from the 2010 Nationwide Inpatient Sample (NIS), from the DHHS Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project (HCUP), it was found that the incremental increase in costs for RBS is as high as $28,814 per patient in the US.[16] This suggests that there can be considerable financial savings for hospitals by reducing even a small percentage of patients with RBS by preventing chest tube clogging after heart surgery.
The result of ineffective evacuation of blood and fluid from a surgical or traumatic wound after surgery has also been reported in the literature under other names, including retained blood complications (RBC), retained blood complex (RBC) and failure to drain.
References
- ↑ Light, R.W.; Rogers, J.T.; Moyers, J.P.; et al. (2002). "Prevalence and Clinical Course of Pleural Effusions at 30 Days after Coronary Artery and Cardiac Surgery". Am J Respir Crit Care Med. 166 (12): 1567–1571. doi:10.1164/rccm.200203-184OC.
- 1 2 Christensen, M.C.; Dziewior, F.; Kempel, A.; von Heymann, C. (2012). "Increased chest tube drainage is independently associated with adverse outcome after cardiac surgery". J Cardiothorac Vasc Anesth. 26 (1): 46–51. doi:10.1053/j.jvca.2011.09.021. PMID 22100857.
- ↑ Light, R.W. (2001). "Pleural effusions following cardiac injury and coronary artery bypass graft surgery". Semin Respir Crit Care Med. 22 (6): 657–664. doi:10.1055/s-2001-18802. PMID 16088710.
- ↑ Light, R.W. (2002). "Pleural effusions after coronary artery bypass graft surgery". Curr Opin Pulm Med. 8 (4): 308–311.
- ↑ Light, R.W.; Rogers, J.T.; Cheng, D.; Rodriguez, R.M. (1999). "Large pleural effusions occurring after coronary artery bypass grafting". Ann Intern Med. Cardiovascular Surgery Associates, PC. 130 (11): 891–896. doi:10.7326/0003-4819-130-11-199906010-00004. PMID 10375337.
- ↑ Ikaheimo, M.J.; Huikuri, H.V.; Airaksinen, K.E.; Korhonen, U.R.; Linnaluoto, M.K.; Tarkka, M.R.; Takkunen, J.T. (1988). "Pericardial effusion after cardiac surgery: incidence, relation to the type of surgery, antithrombotic therapy, and early coronary bypass graft patency". Am Heart J. 116 (1 Pt 1): 97–102. doi:10.1016/0002-8703(88)90255-4. PMID 3260740.
- ↑ Analysis performed by Fletcher Spaght, Inc. using Heart Surgery ICD-9 codes to extract data from the 2010 Nationwide Inpatient Sample (NIS) from the DHHS Agency for Healthcare Research and Quality (AHRQ) and the Healthcare Cost and Utilization Project (HCUP).
- ↑ Clark, G.; Licker, M.; Bertin, D.; Spiliopoulos, A. (2007). "Small size new silastic drains: life-threatening hypovolemic shock after thoracic surgery associated with a non-functioning chest tube". Eur J Cardiothorac Surg. 31 (3): 566–568. doi:10.1016/j.ejcts.2006.12.010. PMID 17215136.
- ↑ Karimov, J.H.; Gillinov, A.M.; Schenck, L.; et al. (2013). "Incidence of chest tube clogging after cardiac surgery: a single-centre prospective observational study". Eur J Cardiothorac Surg. 44 (6): 1029–1036. doi:10.1093/ejcts/ezt140. PMID 23520232.
- ↑ Balzer F, von Heymann C, Boyle EM, Wernecke KD, Grubitzsch H, Sander M (2016). "Impact of retained blood requiring reintervention on outcomes after cardiac surgery". J Thorac Cardiovasc Surg. doi:10.1016/j.jtcvs.2016.03.086. PMID 27210474. pii: S0022-5223(16)30109-X.
- 1 2 Boyle E.M., Gillinov A.M., Cohn W.E.; et al. (September–October 2015). "Retained Blood Syndrome After Cardiac Surgery: A New Look at an Old Problem". Innovations: Technology & Techniques in Cardiothoracic & Vascular Surgery. 10 (5): 296–303. doi:10.1097/IMI.0000000000000200.
- ↑ Imazio, M (2013). "Postpericardiotomy syndrome: a proposal for diagnostic criteria". J Cardiovasc Med. 14: 351–353. doi:10.2459/JCM.0b013e328353807d. PMID 22526225.
- ↑ Dressler, W (1956). "A post-myocardial infarction syndrome; preliminary report of a complication resembling idiopathic, recurrent, benign pericarditis". J Am Med Assoc. 160: 1379–1383. doi:10.1001/jama.1956.02960510005002. PMID 13306560.
- ↑ Karimov, J.H. (Dec 2013). "Incidence of chest tube clogging after cardiac surgery: a single-centre prospective observational study". Eur J Cardiothorac Surg. 44 (6): 1029–36. doi:10.1093/ejcts/ezt140.
- ↑ Sirch, J (2015). "Active Clearance of Chest Drainage Catheters Reduces Retained Blood". J Thorac Cardiovasc Surg. doi:10.1016/j.jtcvs.2015.10.015. PMID 26611748.
- ↑ 2010 Nationwide Inpatient Sample (NIS), from the DHHS Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project (HCUP). Data extracted using ICD-9 codes from over 313,000 US adult heart surgery patients. Analysis performed by Fletcher Spaght, Inc.