Retinoblastoma
| Retinoblastoma | |
|---|---|
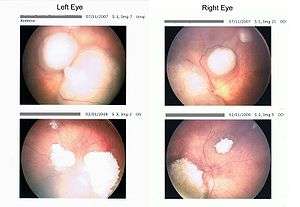 | |
| Rb tumors taken with a retinoscan before and during chemotherapy | |
| Classification and external resources | |
| Specialty | Oncology |
| ICD-10 | C69.2 |
| ICD-9-CM | 190.5 |
| ICD-O | M9510/3 |
| OMIM | 180200 |
| DiseasesDB | 11434 |
| MedlinePlus | 001030 |
| eMedicine | oph/346 |
| Patient UK | Retinoblastoma |
| MeSH | D012175 |
| GeneReviews | |
Retinoblastoma (Rb) is a rare form of cancer that rapidly develops from the immature cells of a retina, the light-detecting tissue of the eye. It is the most common malignant cancer of the eye in children, and it is almost exclusively found in young children.[1]
Though most children survive this cancer, they may lose their vision in the affected eye(s) or need to have the eye removed.
Almost half of children with retinoblastoma have a hereditary genetic defect associated with retinoblastoma. In other cases, it is caused by a congenital mutation in the chromosome 13 gene, 13q14.
Classification
There are two forms of the disease, a heritable form and non-heritable form (all cancers are considered genetic in that mutations of the genome are required for their development, but this does not imply that they are heritable, or transmitted to offspring). Approximately 55% of children with retinoblastoma have the non-heritable form. If there is no history of the disease within the family, the disease is labeled "sporadic", but this does not necessarily indicate that it is the non-heritable form. Bilateral retinoblastomas are commonly heritable, while unilateral retinoblastomas are commonly non-heritable.
In about two-thirds of cases,[2] only one eye is affected (unilateral retinoblastoma); in the other third, tumors develop in both eyes (bilateral retinoblastoma). The number and size of tumours on each eye may vary. In certain cases, the pineal gland or the suprasellar or parasellar region (or in very rare cases other midline intracranial locations) is also affected (trilateral retinoblastoma). The position, size and quantity of tumours are considered when choosing the type of treatment for the disease.
Signs and symptoms

The most common and obvious sign of retinoblastoma is an abnormal appearance of the retina as viewed through the pupil, the medical term for which is leukocoria, also known as amaurotic cat's eye reflex.[1] Other signs and symptoms include deterioration of vision, a red and irritated eye with glaucoma, and faltering growth or delayed development. Some children with retinoblastoma can develop a squint,[3] commonly referred to as "cross-eyed" or "wall-eyed" (strabismus). Retinoblastoma presents with advanced disease in developing countries and eye enlargement is a common finding.
Depending on the position of the tumors, they may be visible during a simple eye exam using an ophthalmoscope to look through the pupil. A positive diagnosis is usually made only with an examination under anesthetic (EUA). A white eye reflection is not always a positive indication of retinoblastoma and can be caused by light being reflected badly or by other conditions such as Coats' disease.
The presence of the photographic fault red eye in only one eye and not in the other may be a sign of retinoblastoma. A clearer sign is "white eye" or "cat's eye" (leukocoria).[4]
Cause
Mutation of genes, found in chromosomes, can affect the way in which cells grow and develop within the body.[5] Alterations in RB1 or MYCN can give rise to retinoblastoma.
RB1
In children with the heritable genetic form of retinoblastoma there is a mutation in the RB1 gene on chromosome 13. RB1 was the first tumor suppressor gene cloned.[5] Although RB1 interacts with over 100 cell proteins,[5] its negative regulator effect on the cell cycle principally arises from binding and inactivation of the transcription factor E2F, thus repressing the transcription of genes which are required for the S phase.[5]
The defective RB1 gene can be inherited from either parent; in some children, however, the mutation occurs in the early stages of fetal development. The expression of the RB1 allele is autosomal dominant with 90% penetrance.
Inherited forms of retinoblastomas are more likely to be bilateral. In addition, inherited uni- or bilateral retinoblastomas may be associated with pineoblastoma and other malignant midline supratentorial primitive neuroectodermal tumors (PNET) with a dismal outcome; retinoblastoma concurrent with a PNET is known as trilateral retinoblastoma.[6] A recent meta-analysis has shown that survival of trilateral retinoblastoma has increased substantially over the last decades.[7]
The development of retinoblastoma can be explained by the two-hit model. According to the two-hit model, both alleles need to be affected, so two events are necessary for the retinal cell or cells to develop into tumors. The first mutational event can be inherited (germline or constitutional) which will then be present in all cells in the body. The second “hit” results in the loss of the remaining normal allele (gene) and occurs within a particular retinal cell.[8] In the sporadic, nonheritable form of retinoblastoma, both mutational events occur within a single retinal cell after fertilization (somatic events); sporadic retinoblastoma tends to be unilateral.
Several methods have been developed to detect the RB1 gene mutations.[9][10] Attempts to correlate gene mutations to the stage at presentation have not shown convincing evidence of a correlation.[11]
MYCN
Somatic amplification of the MYCN oncogene is responsible for some cases of non-hereditary, early-onset, aggressive, unilateral retinoblastoma. Although MYCN amplification accounted for only 1.4% of retinoblastoma cases, researchers identified it in 18% of infants diagnosed at less than 6 months of age. Median age at diagnosis for MYCN retinoblastoma was 4.5 months, compared with 24 months for those who had non-familial unilateral disease with two RB1 gene mutations.[12]
Diagnosis
Screening for retinoblastoma should be part of a "well baby" screening for newborns during the first three months of life, to include:
- The red reflex: checking for a normal reddish-orange reflection from the eye's retina with an ophthalmoscope or retinoscope from approximately 30 cm / 1 foot, usually done in a dimly lit or dark room.
- The corneal light reflex / Hirschberg test: checking for symmetrical reflection of beam of light in the same spot on each eye when a light is shined into each cornea, to help determine whether the eyes are crossed.
- Eye examination: checking for any structural abnormalities.
- Bryan Shaw helped develop a smart-phone app that can detect leukocoria in photos.[13]
Differential diagnosis
- 1. Persistent hyperplastic primary vitreous (PHPV): congenital developmental anomaly of the eye resulting from failure of the embryological, primary vitreous and hyaloid vasculature to regress, whereby the eye is shorter, develops a cataract, and may present with whitening of the pupil.
- 2. Coats disease: a typically unilateral disease characterised by abnormal development of blood vessels behind the retina, leading to blood vessel abnormalities in the retina and retinal detachment to mimic retinoblastoma.
- 3. Toxocara canis: an infectious disease of the eye associated with exposure to infected puppies, which causes a retinal lesion leading to retinal detachment.
- 4. Retinopathy of prematurity (ROP): associated with low birth weight infants who receive supplemental oxygen in the period immediately after birth, it involves damage to the retinal tissue and may lead to retinal detachment.
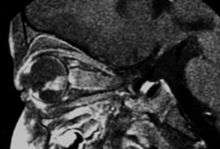
If the eye examination is abnormal, further testing may include imaging studies, such as computerized tomography (CT), magnetic resonance imaging (MRI), and ultrasound.[14] CT and MRI can help define the structure abnormalities and reveal any calcium depositions. Ultrasound can help define the height and thickness of the tumor. Bone marrow examination or lumbar puncture may also be done to determine any metastases to bones or the brain.
Morphology
Gross and microscopic appearances of retinoblastoma are identical in both hereditary and sporadic types. Macroscopically, viable tumor cells are found near blood vessels, while zones of necrosis are found in relatively avascular areas. Microscopically, both undifferentiated and differentiated elements may be present. Undifferentiated elements appear as collections of small, round cells with hyperchromatic nuclei; differentiated elements include Flexner-Wintersteiner rosettes, Homer Wright rosettes,[15] and fleurettes from photoreceptor differentiation.[16]
 Drawing of a large retinoblastoma
Drawing of a large retinoblastoma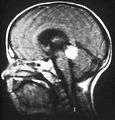 Aspect of trilateral retinoblastoma on MRI
Aspect of trilateral retinoblastoma on MRI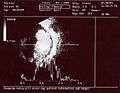 An ocular ultrasound of a large retinoblastoma tumor within the eye of a three-year-old boy
An ocular ultrasound of a large retinoblastoma tumor within the eye of a three-year-old boy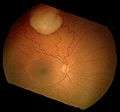 Funduscopic finding of a retinoblastoma
Funduscopic finding of a retinoblastoma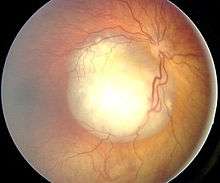 Ocular fundus aspect of retinoblastoma
Ocular fundus aspect of retinoblastoma Gross pathology of retinoblastoma tumor in enucleated eye of three-year-old female
Gross pathology of retinoblastoma tumor in enucleated eye of three-year-old female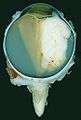 Large exophytic white tumor with foci of calcification producing total exudative retinal detachment
Large exophytic white tumor with foci of calcification producing total exudative retinal detachment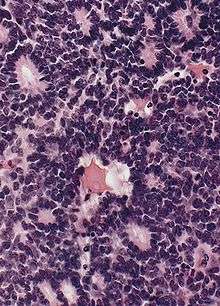 Flexner-Wintersteiner rosettes in Retinoblatoma
Flexner-Wintersteiner rosettes in Retinoblatoma- Retinoblastoma, 400 X magnification
- Crystal structure of the Retinoblastoma tumour suppressor protein bound to E2F peptide Polymer.
Genetic testing
Identifying the RB1 gene mutation that led to a child's retinoblastoma can be important in the clinical care of the affected individual and in the care of (future) siblings and offspring.It may run in the family.
- Bilaterally affected individuals and 13-15% of unilaterally affected individuals,[17][18] are expected to show an RB1 mutation in blood. By identifying the RB1 mutation in the affected individual, (future) siblings, children, and other relatives can be tested for the mutation; if they do not carry the mutation, child relatives are not at risk of retinoblastoma so need not undergo the trauma and expense of examinations under anaesthetic.[19] For the 85% of unilaterally affected patients found not to carry either of their eye tumor RB1 mutations in blood, neither molecular testing nor clinical surveillance of siblings is required.
- If the RB1 mutation of an affected individual is identified, amniotic cells in an at-risk pregnancy can be tested for the family mutation; any fetus that carries the mutation can be delivered early, allowing early treatment of any eye tumors, leading to better visual outcomes.[19]
- For cases of unilateral retinoblastoma where no eye tumor is available for testing, if no RB1 mutation is detected in blood after high sensitivity molecular testing (i.e. >93% RB1 mutation detection sensitivity), the risk of a germline RB1 mutation is reduced to less than 1%,[18] a level at which only clinic examination (and not examinations under anaesthetic) is recommended for the affected individual and their future offspring (National Retinoblastoma Strategy, Canadian Guidelines for Care).[20]
Treatment

The priority of retinoblastoma treatment is to preserve the life of the child, then to preserve vision, and then to minimize complications or side effects of treatment. The exact course of treatment will depend on the individual case and will be decided by the ophthalmologist in discussion with the paediatric oncologist.[21] Children with involvement of both eyes at diagnosis usually require multimodality therapy (chemotherapy, local therapies)
The various treatment modalities for retinoblastoma includes:[22]
- Enucleation of the eye – Most patients with unilateral disease present with advanced intraocular disease and therefore usually undergo enucleation, which results in a cure rate of 95%. In bilateral Rb, enucleation is usually reserved for eyes that have failed all known effective therapies or without useful vision.
- External beam radiotherapy (EBR) – The most common indication for EBR is for the eye in a young child with bilateral retinoblastoma who has active or recurrent disease after completion of chemotherapy and local therapies. However, patients with hereditary disease who received EBR therapy are reported to have a 35% risk of second cancers.[23]
- Brachytherapy – Brachytherapy involves the placement of a radioactive implant (plaque), usually on the sclera adjacent to the base of a tumor. It used as the primary treatment or, more frequently, in patients with small tumors or in those who had failed initial therapy including previous EBR therapy.
- Thermotherapy – Thermotherapy involves the application of heat directly to the tumor, usually in the form of infrared radiation. It is also used for small tumors
- Laser photocoagulation – Laser photocoagulation is recommended only for small posterior tumors. An argon or diode laser or a xenon arc is used to coagulate all the blood supply to the tumor.
- Cryotherapy – Cryotherapy induces damage to the vascular endothelium with secondary thrombosis and infarction of the tumor tissue by rapidly freezing it. Cryotherapy may be used as primary therapy for small peripheral tumors or for small recurrent tumors previously treated with other methods.
- Systemic chemotherapy – Systemic chemotherapy has become forefront of treatment in the past decade, in the search of globe preserving measures and to avoid the adverse effects of EBR therapy. The common indications for chemotherapy for intraocular retinoblastoma include tumors that are large and that cannot be treated with local therapies alone in children with bilateral tumors. It is also used in patients with unilateral disease when the tumors are small but cannot be controlled with local therapies alone.
- Intra-arterial chemotherapy – Chemotherapeutic drugs are administered locally via a thin catheter threaded through the groin, through the aorta and the neck, directly into the optic vessels.[24]
- Nano-particulate chemotherapy – To reduce the adverse effects of systemic therapy, subconjuctival (local) injection of nanoparticle carriers containing chemotherapeutic agents (carboplatin) has been developed which has shown promising results in the treatment of retinoblastoma in animal models without adverse effects.[25][26]
Prognosis
In the developed world, retinoblastoma has one of the best cure rates of all childhood cancers (95-98%), with more than nine out of every ten sufferers surviving into adulthood. In the UK, around 40 to 50 new cases are diagnosed each year.
Good prognosis depends upon early presentation of the child in health facility.[27][28] Late presentation of the child in hospital is associated with poor prognosis.[29]
Survivors of hereditary retinoblastoma have a higher risk of developing other cancers later in life.
Epidemiology
Retinoblastoma presents with cumulative lifetime incidence rate of 1 case of retinoblastoma per 18000 to 30000 live births worldwide.[30] A higher incidence is noted in developing countries, this has been attributed to lower socioeconomic status and the presence of human papilloma virus sequences in the retinoblastoma tissue.[31]
Almost 80% of children with retinoblastoma are diagnosed before 3 years of age and diagnosis in children above 6 years of age is extremely rare.[32] In the UK, bilateral cases usually present within 14–16 months, while diagnosis of unilateral cases peaks between 24 and 30 months.
See also
References
- 1 2 American Cancer Society (2003). "Chapter 85. Neoplasms of the Eye". Cancer Medicine. Hamilton, Ontario: BC Decker Inc. ISBN 1-55009-213-8.
- ↑ MacCarthy A, Birch JM, Draper GJ, et al. (January 2009). "Retinoblastoma in Great Britain 1963-2002". Br J Ophthalmol. 93 (1): 33–7. doi:10.1136/bjo.2008.139618. PMID 18838413.
- ↑ A. R. Elkington; P. T. Khaw (1988). "ABC of eyes. Squint". BMJ. 297 (6648): 608–611. doi:10.1136/bmj.297.6648.608. PMC 1834556
 . PMID 3139234.
. PMID 3139234. - ↑ Introduction to White Eye, Daisy's Eye Cancer Fund.
- 1 2 3 4 Du W, Pogoriler J (August 2006). "Retinoblastoma family genes". Oncogene. 25 (38): 5190–200. doi:10.1038/sj.onc.1209651. PMC 1899835
 . PMID 16936737.
. PMID 16936737. - ↑ Kivelä T (June 1999). "Trilateral retinoblastoma: a meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma". Journal of Clinical Oncology. 17 (6): 1829–37. PMID 10561222.
- ↑ de Jong MC, et al. (August 2014). "Trilateral retinoblastoma: a systematic review and meta-analysis". The Lancet Oncology. 15: 1157–1167. doi:10.1016/s1470-2045(14)70336-5. PMID 25126964.
- ↑ Harbour JW,DeanDC. Rb function in cell-cycle regulation and apoptosis" Nat Cell Biol 2000;94:E65–E67
- ↑ Parsam VL, Kannabiran C, Honavar S, et al. (December 2009). "A comprehensive, sensitive and economical approach for the detection of mutations in the RB1 gene in retinoblastoma". J. Genet. 88 (4): 517–27. doi:10.1007/s12041-009-0069-z. PMID 20090211.
- ↑ Lohmann DR, Gallie BL (2010). "Retinoblastoma". GeneReviews. Seattle, WA: University of Washington. PMID 20301625.
- ↑ Parsam Ali MJ, Parsam VL, Honavar SG, et al. (2010). "RB1 gene mutations in retinoblastoma and its clinical correlation". Saudi Journal of Ophthalmology. 24 (4): 119–123. doi:10.1016/j.sjopt.2010.05.003. PMID 23960888.
- ↑ Lewis R (March 19, 2013). "Some Aggressive Retinoblastomas Lack RB1 Mutations". Medscape online.
- ↑ National Public Radio, Morning Edition, October 31, 2014; http://www.npr.org/blogs/health/2014/10/31/359568507/look-here-phone-app-checks-photos-for-eye-disease
- ↑ de Jong MC, de Graaf P, Noij DP, Göricke S, Maeder P, Galluzzi P, Brisse HJ, Moll AC, Castelijns JA (May 2014). "Diagnostic performance of magnetic resonance imaging and computed tomography for advanced retinoblastoma: a systematic review and meta-analysis". Ophthalmology. 121 (5): 1109–18. doi:10.1016/j.ophtha.2013.11.021. PMID 24589388.
- ↑ Lee,, K. Weng Sehu,... William R. (2005). Ophthalmic pathology : an illustrated guide for clinicans. Malden: Blackwell publ. p. 262. ISBN 978-0-7279-1779-9.
- ↑ Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. Seventh Edition. Philadelphia: Elsevier Saunders, 2005, p. 1442.
- ↑ Schüler A, Weber S, Neuhäuser M, et al. (March 2005). "Age at diagnosis of isolated unilateral retinoblastoma does not distinguish patients with and without a constitutional RB1 gene mutation but is influenced by a parent-of-origin effect". Eur J Cancer. 41 (5): 735–40. doi:10.1016/j.ejca.2004.12.022. PMID 15763650.
- 1 2 Rushlow D, Piovesan B, Zhang K, et al. (May 2009). "Detection of mosaic RB1 mutations in families with retinoblastoma". Hum Mutat. 30 (5): 842–51. doi:10.1002/humu.20940. PMID 19280657.
- 1 2 Richter S, Vandezande K, Chen N, et al. (December 2002). "Sensitive and efficient detection of RB1 gene mutations enhances care for families with retinoblastoma". Am J Hum Genet. 72 (2): 253–69. doi:10.1086/345651. PMC 379221
 . PMID 12541220.
. PMID 12541220. - ↑ Canadian Ophthalmological Society' (December 2009). "National Retinoblastoma Strategy Canadian Guidelines for Care; Genetic Analysis" (PDF). Canadian Journal of Ophthalmology. 44 (suppl.2): S17–S22. doi:10.3129/i09-194.
- ↑ Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R (October 2007). "Retinoblastoma: review of current management". Oncologist. 12 (10): 1237–46. doi:10.1634/theoncologist.12-10-1237. PMID 17962617.
- ↑ Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management" Oncologist 2007;12(10) 1237–1246
- ↑ Roarty JD, McLean IW, Zimmerman LE. Incidence of second neoplasms in patients with bilateral retinoblastoma" Ophthalmology 1988;95:1583–1587
- ↑ Shields, CL; Ramasubramanian, A; Rosenwasser, R; Shields, JA (September 2009). "Superselective catheterization of the ophthalmic artery for intraarterial chemotherapy for retinoblastoma.". Retina (Philadelphia, Pa.). 29 (8): 1207–9. doi:10.1097/IAE.0b013e3181b4ce39. PMID 19734768.
- ↑ Shome D, Poddar N, Sharma V, et al. (2009). "Does a Nanomolecule of Carboplatin Injected Periocularly Help in Attaining Higher Intravitreal Concentrations?". Investigative Ophthalmology & Visual Science. 50 (12): 5896–900. doi:10.1167/iovs.09-3914.
- ↑ Kang SJ, Durairaj C, Kompella UB, et al. (2009). "Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma". Archives of Ophthalmology. 127 (8): 1043–7. doi:10.1001/archophthalmol.2009.185.
- ↑ Syed Imtiaz Ali Shah: Concise Ophthalmology. 4th ed. Paramount B (Pvt.) Ltd. 2014: 80-81
- ↑ http://www.paramountbooks.com.pk/LoginIndex.asp?title=Concise-Ophthalmology-(pb)-2014&Isbn=9789696370017&opt=3&sUBcAT=06
- ↑ Partab Rai, Imtiaz Ali Shah, Ashok Kumar Nasrani, Mahesh Kumar Lohana, Muhammad Khan Memon, Manzoor Ahmed Memon: Too late presentation of 53 patients with retinoblastoma:a big challenge: International J Ophthalmology 2009, Vol. 9 No. 2 ; p.227-230.
- ↑ Abramson D.H.; Schefler A.C. (2004). "Update on retinoblastoma". Retina. 24: 828–48. doi:10.1097/00006982-200412000-00002.
- ↑ Orjuela M, Castaneda VP, Ridaura C, et al. (2000). "Presence of human papilloma virus in tumor tissue from children with retinoblastoma: An alternative mechanism for tumor development". Clin Cancer Res. 6: 4010–4016.
- ↑ Abramson DH, Frank CM, Susman M, et al. (1998). "Presenting signs of retinoblastoma". J Pediatr. 132: 505–508. doi:10.1016/s0022-3476(98)70028-9.
External links
- Retinoblastoma at Dana-Farber/Boston Children's Cancer and Blood Disorders Center
- Retinoblastoma at National Cancer Institute
- Retinoblastoma information from MedlinePlus
- retinoblastoma at NIH/UW GeneTests
- RB1 Mutation Database
- Retinoblastoma at DMOZ