SYBR Green I
 | |
| Names | |
|---|---|
| IUPAC name
N',N'-dimethyl-N-[4-[(E)-(3-methyl-1,3-benzothiazol-2-ylidene)methyl]-1-phenylquinolin-1-ium-2-yl]-N-propylpropane-1,3-diamine | |
| Identifiers | |
| 163795-75-3 | |
| ChEMBL | ChEMBL1198924 |
| PubChem | 10436340 |
| Properties | |
| C32H37N4S+ | |
| Molar mass | 509.73 g·mol−1 |
| Solubility | Soluble in Dimethylsulfoxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
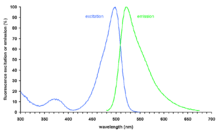
SYBR Green I (SG) is an asymmetrical cyanine dye[1] used as a nucleic acid stain in molecular biology. The SYBR family of dyes is produced by Molecular Probes Inc., a wholly owned subsidiary of Life Technologies Corporation. SYBR Green I binds to DNA. The resulting DNA-dye-complex absorbs blue light (λmax = 497 nm) and emits green light (λmax = 520 nm). The stain preferentially binds to double-stranded DNA, but will stain single-stranded DNA with lower performance. SYBR Green can also stain RNA with a lower performance than DNA.
Uses
SYBR Green finds usage in several areas of biochemistry and molecular biology. It is used as a dye for the quantification of double stranded DNA in some methods of quantitative PCR.[2] It is also used to visualise DNA in gel electrophoresis. Higher concentrations of SYBR Green can be used to stain agarose gels in order to visualise the DNA present. In addition to labelling pure nucleic acids SYBR Green can also be used for labelling of DNA within cells for flow cytometry and fluorescence microscopy. In these cases RNase treatment may be required to reduce background from RNA in the cells.
Safety
SYBR Green I is marketed as a replacement for the potential human mutagen ethidium bromide, as both safer to work with and free from the complex waste disposal issues of ethidium. However any small molecule capable of binding DNA with high affinity is a possible carcinogen, including SYBR Green.
In a study using the Ames test, which measures the ability of chemicals to cause mutations, when assayed at the same concentration there was no clear pattern in the relative mutagenicities of ethidium bromide and SYBR green I.[3] Ethidium bromide was more mutagenic at higher concentrations.
Similar cyanine dyes
- SYBR Green II
- SYBR Gold
- YO (Oxazole Yellow)
- TO (Thiazole Orange)
- PG (PicoGreen)
See also
Notes and references
- ↑ Zipper H; Brunner H; Bernhagen J; Vitzthum F (2004). "Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications". Nucleic Acids Research. 32 (12): e103. doi:10.1093/nar/gnh101. PMC 484200
 . PMID 15249599.
. PMID 15249599. - ↑ Mackay IM; Arden KE; Nitsche A (March 2002). "Real-time PCR in virology". Nucleic Acids Res. 30 (6): 1292–305. doi:10.1093/nar/30.6.1292. PMC 101343
 . PMID 11884626.
. PMID 11884626. - ↑ Singer VL; Lawlor TE; Yue S (February 1999). "Comparison of SYBR Green I nucleic acid gel stain mutagenicity and ethidium bromide mutagenicity in the Salmonella/mammalian microsome reverse mutation assay (Ames test)". Mutation research. 439 (1): 37–47. doi:10.1016/s1383-5718(98)00172-7. PMID 10029672.
