Stollé synthesis
The Stollé synthesis is a series of chemical reactions that produce oxindoles from anilines and α-haloacid chlorides (or oxalyl chloride).[1][2][3][4]
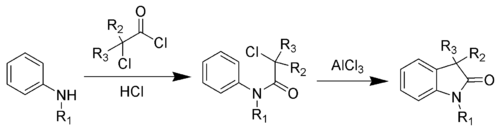
The first step is an amide coupling, while the second step is a Friedel–Crafts reaction.[5][6] An improved procedure has been developed.[7][8]
See also
References
- ↑ Stollé, R. Ber. (1913). "Über eine neue Methode zur DarstellungN-substituierter Isatine. (Vorläufige Mitteilung)". Berichte der deutschen chemischen Gesellschaft. 46 (3): 3915. doi:10.1002/cber.191304603186.
- ↑ Stollé, R. (1914). "Über Phenyl-oxindol". Ber. 47 (2): 2120. doi:10.1002/cber.191404702112.
- ↑ Stollé, R. (1923). "Über N-substituierte Oxindole und Isatine". J. Prakt. Chem. 105: 137. doi:10.1002/prac.19221050111.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help) - ↑ Stollé, R. (1930). "Über N-substituierte Oxindole und Isatine". J. Prakt. Chem. 128: 1. doi:10.1002/prac.19301280101.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help) - ↑ Sumpter, W. C. (1944). "The Chemistry of Isatin". Chem. Rev. 34 (3): 396. doi:10.1021/cr60109a003.
- ↑ Sumpter, W. C. (1945). "The Chemistry of Oxindole". Chem. Rev. 37 (3): 443–479. doi:10.1021/cr60118a003.
- ↑ Julian; Pikl (1935). "Studies in the Indole Series. IV. The Synthesis of d,l-Eserethole". J. Am. Chem. Soc. 57 (3): 563. doi:10.1021/ja01306a053.
- ↑ Rutenberg, M. W.; Horning, E. C. (1963). "1-Methyl-3-ethyloxindole". Org. Synth.; Coll. Vol., 4, p. 620
This article is issued from Wikipedia - version of the 6/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.