Thrombodynamics test
Thrombodynamics is a method for blood coagulation monitoring and anticoagulant control. This test is based on imitation of coagulation processes occurring in vivo, is sensitive both to pro- and anticoagulant changes in the hemostatic balance. Highly sensitive to thrombosis.
The method was developed in the Physical Biochemistry Laboratory under the direction of Prof. Fazly Ataullakhanov.
Technology description
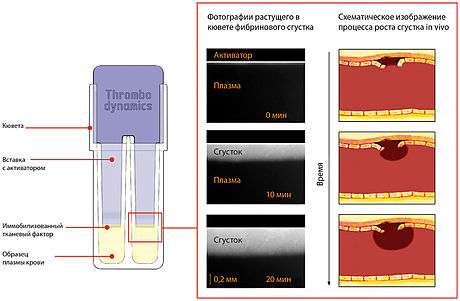
Thrombodynamics designed to investigate the in vitro spatial-temporal dynamics of blood coagulation initiated by localized coagulation activator under conditions similar to the conditions of the blood clotting in vivo. Thrombodynamics takes into account the spatial heterogeneity trombodinamiki processes in blood coagulation. The test is performed without mixing in a thin layer of plasma.
The measurement cuvette with the blood plasma sample is placed inside the water thermostat. Clotting starts when activator with immobilized TF is immersed into the cuvette. The clot then propagates from the activating surface into the bulk of plasma. Image of growing clot is registered via the CCD camera using a time-lapse microscopy mode in scattered light and then parameters of coagulation are calculated on the computer. Thrombodynamics analyser T-2 device also supports measurement of spatial dynamics of thrombin propagation during the process of clot growth via usage of the fluorogenic substrate for thrombin. Blood plasma sample is periodically irradiated with the excitation light and the emission of the fluorophore is registered by CCD camera.
Mathematical methods are used to restore spatio-temporal distribution of the thrombin from the fluorophore signal. This experimental model worked well in research and has demonstrated good sensitivity to various disorders of the coagulation system.
Features
- Observing spatial growth of fibrin clots in vitro
- Measuring thrombin generation as a function of space and time during thrombus formation
- Time- and space- resolved imaging of other active coagulation factors
- High sensitivity to both pro- and anticoagulant changes in the hemostatic balance
Applications
- Prothrombotic disorders
- Hypercoagulation in the perioperative period, DIC, cancer patients, patients with thrombotic risk factors
- Diagnostics of coagulopathy
- Monitoring of anticoagulant therapy
- Individual therapy planning
- Bleeding disorders
- Hemophilia A and B, DIC, blood loss, anticoagulant overdose
- Diagnostics of coagulopathy
- Monitoring of replacement therapy
- Identification of coagulopathy pathogenesis
- Development of drugs and biomaterials
See also
References
- ↑ Soshitova NP, Karamzin SS, Fadeeva OA, et al. (2012). "Predicting prothrombotic tendencies in sepsis using spatial clot growth dynamics". Blood Coagul Fibrinolysis. 6 (23): 498–507. doi:10.1097/MBC.0b013e328352e90e. PMID 22688554.
- ↑ Parunov LA, Fadeeva OA, Balandina AN, et al. (2011). "Improvement of spatial fibrin formation by the anti-TFPI aptamer BAX499: changing clot size by targeting extrinsic pathway initiation.". Thromb Haemost. 22 (Jun): 1825–34. doi:10.1111/j.1538-7836.2011.04412.x. PMID 21696535.
- ↑ Ovanesov MV, Panteleev MA, Sinauridze EI, et al. (2008). "Mechanisms of action of recombinant activated factor VII in the context of tissue factor concentration and distribution.". Blood Coagul Fibrinolysis. 8 (19): 743–755. doi:10.1097/MBC.0b013e3283104093. PMID 19002040.
- ↑ Ovanesov M.V.; Krasotkina J.V.; Ul’yanova L.I.; et al. (2002). "Hemophilia A and B are associated with abnormal spatial dynamics of clot growth.". Biochim Biophys Acta. 1572: 45–57. doi:10.1016/s0304-4165(02)00278-7.
- ↑ Ovanesov M.V.; Lopatina E.G.; Saenko E.L.; et al. (2003). "Effect of factor VIII on tissue factor-initiated spatial clot growth.". Thromb Haemost. 89: 235–242.
- ↑ Ovanesov M.V.; Ananyeva N.M.; Panteleev M.A.; et al. (2005). "Initiation and propagation of coagulation from tissue factor bearing cell monolayers to plasma: initiator cells do not regulate spatial growth rate.". J Thromb Haemost. 2 (3): 321–331.
- ↑ Panteleev M.A.; Ovanesov M.V.; Kireev D.A; et al. (2006). "Spatial propagation and localization of blood coagulation are regulated by intrinsic and protein C pathways, respectively.". Biophys J. 90: 1489–1500. doi:10.1529/biophysj.105.069062.