Trypan blue
 | |
| Names | |
|---|---|
| IUPAC name
(3Z,3'Z)-3,3'-[(3,3'-dimethylbiphenyl-4,4'-diyl)di(1Z)hydrazin-2-yl-1-ylidene]bis(5-amino-4-oxo-3,4-dihydronaphthalene-2,7-disulfonic acid) | |
| Identifiers | |
| 72-57-1 (tetrasodium salt) | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:78897 |
| ChEMBL | ChEMBL1640 |
| ChemSpider | 10482308 |
| ECHA InfoCard | 100.000.715 |
| KEGG | C19307 |
| PubChem | 5904246 |
| UNII | I2ZWO3LS3M |
| |
| |
| Properties | |
| C34H28N6O14S4 | |
| Molar mass | 872.88 |
| Appearance | deep blue in aqueous solution[1] |
| Melting point | > 300 °C (572 °F; 573 K) |
| <0.1 mg/mL in water [2] | |
| Solubility | 20 mg/mL in methyl Cellosolve, and 0.6 mg/mL in ethanol |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
6200 mg/kg (oral, rat) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Trypan blue is a vital stain used to selectively colour dead tissues or cells blue. It is a diazo dye.
Live cells or tissues with intact cell membranes are not coloured. Since cells are very selective in the compounds that pass through the membrane, in a viable cell trypan blue is not absorbed; however, it traverses the membrane in a dead cell. Hence, dead cells are shown as a distinctive blue colour under a microscope. Since live cells are excluded from staining, this staining method is also described as a dye exclusion method. This dye may be a cause of certain birth defects such as encephalocele.
Background & chemistry
Trypan blue is derived from toluidine, that is, any of several isomeric bases, C14H16N2, derived from toluene. Trypan blue is so-called because it can kill trypanosomes, the parasites that cause sleeping sickness. An analog of trypan blue, suramin is used pharmacologically against trypanosomiasis. Trypan blue is also known as diamine blue and Niagara blue.
The extinction coefficient for trypan blue is 6 x 104 M−1 cm−1 at 607nm in methanol.[3]
Trypan red and Trypan blue were first synthesized by the German scientist Paul Ehrlich in 1904.
Uses of trypan blue
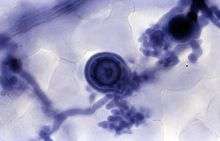
Trypan blue is commonly used in microscopy (for cell counting) and in laboratory mice for assessment of tissue viability.[4] The method cannot distinguish between necrotic and apoptotic cells.
It may be used to observe fungal hyphae[5] and stramenopiles.
Trypan blue is also used in ophthalmic cataract surgery to stain the anterior capsule in the presence of a mature cataract, to aid in visualization, before creating the continuous curvilinear capsulorhexis.
In early 20th century, the existence of a barrier protective toward the brain (blood brain barrier was inferred upon based on the observation that injection of trypan blue to animals led to whole‐body staining except for the brain and spinal cord.
Synonyms
- Azidine Blue 3B
- Benzamine Blue 3B
- Benzo Blue 3B
- Chlorazol Blue 3B
- Diamine Blue 3B
- Dianil Blue H3G
- Direct Blue 14
- Niagara Blue 3B
Further reading
- Chapter "Detection of Caspase Activation Combined with Other Probes of Apoptosis", Eurekah Bioscience Collection, NCBI bookshelf
- Protocol for use of the dye (PDF) from Northwestern University
- Wainwright, M. (December 2010). "Dyes, trypanosomiasis and DNA: a historical and critical review". Biotechnic & Histochemistry. 85 (6): 341–54. doi:10.3109/10520290903297528. PMID 21080764.
References
| Wikimedia Commons has media related to Trypan blue staining. |
- ↑ http://pubchem.ncbi.nlm.nih.gov/compound/9562061#section=Color
- ↑ http://pubchem.ncbi.nlm.nih.gov/compound/9562061#section=Solubility
- ↑ "Sigma-Aldrich, 60% Trypan Blue, Product page". Retrieved 2015-07-15.
- ↑ Strober, W (May 2001). "Trypan blue exclusion test of cell viability.". Current protocols in immunology. Appendix 3: Appendix 3B. doi:10.1002/0471142735.ima03bs21. PMID 18432654.
- ↑ Nowicki, Marcin; et al. (15 May 2013), A simple dual stain for detailed investigations of plant-fungal pathogen interactions, Vegetable Crops Research bulleting, InHort & Versita, doi:10.2478/v10032-012-0016-z, retrieved 2013-05-24