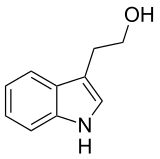
Tryptophol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-(1H-Indol-3-yl)ethanol | |
| Other names | |
| Identifiers | |
| 526-55-6 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:17890 |
| ChEMBL | ChEMBL226545 |
| ChemSpider | 10235 |
| ECHA InfoCard | 100.007.632 |
| PubChem | 10685 |
| |
| Properties | |
| C10H11NO | |
| Molar mass | 161.20 g·mol−1 |
| Melting point | 59 °C (138 °F; 332 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tryptophol is an aromatic alcohol that induces sleep in humans. It is formed in the liver after disulfiram treatment.[1] It is also produced by the trypanosomal parasite in sleeping sickness.
It is also found in wine as a secondary product of alcoholic fermentation. It was first described by Felix Ehrlich in 1912.
Natural occurrences
Tryptophol can be found in Pinus sylvestris needles[2] or seeds.[3]
It is produced by the trypanosomal parasite (Trypanosoma brucei) in sleeping sickness (African trypanosomiasis).[1][4]
It is found in wine[5] or in beer (although at levels of <23 mg/L in Canadian beers[6] as a secondary product of alcoholic fermentation[7] (a product also known as congener) by Saccharomyces cerevisiae.
It is also an autoantibiotic produced by the fungus Candida albicans.[8]
It can also be isolated from the marine sponge Ircinia spiculosa.[9]
Metabolism
Biosynthesis
It was first described by Felix Ehrlich in 1912. Ehrlich demonstrated that yeast attacks the natural amino acids essentially by splitting off carbon dioxide and re-placing the amino group with hydroxyl. By this reaction, tryptophan gives rise to tryptophol.[10] Tryptophan is first deaminated to 3-indolepyruvate. It is then decarboxylated[11] to indole acetaldehyde by indolepyruvate decarboxylase. This latter compound is transformed to tryptophol by alcohol dehydrogenase.[12]
It is formed from tryptophan, along with indole-3-acetic acid in rats infected by Trypanosoma brucei gambiense.[13]
An efficient conversion of tryptophan to indole-3-acetic acid and/or tryptophol can be achieved by some species of fungi in the genus Rhizoctonia.[14]
Biodegradation
In Cucumis sativus (cucumber), the enzymes indole-3-acetaldehyde reductase (NADH) and indole-3-acetaldehyde reductase (NADPH) use tryptophol to form (indol-3-yl)acetaldehyde.[15]
Glycosides
The unicellular alga Euglena gracilis converts exogenous trytophol to two major metabolites: tryptophol galactoside and an unknown compound (a tryptophol ester), and to minor amounts of indole-3-acetic acid, tryptophol acetate and tryptophol glucoside.[16]
Biological effects
Tryptophol and its derivatives 5-hydroxytryptophol and 5-methoxytryptophol, induce sleep in mice. It induces a sleep-like state that lasts less than an hour at the 250 mg/kg dose.[17] These compounds may play a role in physiological sleep mechanisms.[18] It may be a functional analog of serotonin or melatonin, compounds involved in sleep regulation.
Tryptophol shows genotoxicity in vitro.[19]
Tryptophol is a quorum sensing molecule for the yeast Saccharomyces cerevisiae.[20] It is also found in the bloodstream of patients with chronic trypanosomiasis. For that reason, it may be a quorum sensing molecule for the trypanosome parasite.[19]
In the case of trypanosome infection, tryptophol decreases the immune response of the host.[21]
As it is formed in the liver after ethanol ingestion or disulfiram treatment, it is also associated with the study of alcoholism.[1][17] Pyrazole and ethanol have been shown to inhibit the conversion of exogenous tryptophol to indole-3-acetic acid and to potentiate the sleep-inducing hypothermic effects of tryptophol in mice.[22]
It is a growth promoter of cucumber hypocotyl segments.[23] The auxinic action in terms of embryo formation is even better for tryptophol arabinoside on Cucurbita pepo hypocotyl fragments.[24]
Precursor for synthesis of other compounds
Tryptophol has been used as the starting material in the synthesis of DMT[25] and indoramin.
See also
References
- 1 2 3 Cornford, E. M.; Bocash, W. D.; Braun, L. D.; Crane, P. D.; Oldendorf, W. H.; MacInnis, A. J. (1979). "Rapid distribution of tryptophol (3-indole ethanol) to the brain and other tissues". Journal of Clinical Investigation. 63 (6): 1241–1248. doi:10.1172/JCI109419. PMC 372073
 . PMID 447842.
. PMID 447842. - ↑ Sandberg, Göran (1984). "Biosynthesis and metabolism of indole-3-ethanol and indole-3-acetic acid by Pinus sylvestris L. Needles". Planta. 161 (5): 398–403. doi:10.1007/BF00394569.
- ↑ Sandberg, Goran; Ernstsen, Arild; Hamnede, Marianne (1987). "Dynamics of indole-3-acetic acid and indole-3-ethanol during development and germination of Pinus sylvestris seeds". Physiologia Plantarum. 71 (4): 411–418. doi:10.1111/j.1399-3054.1987.tb02876.x.
- ↑ Richard Seed, John; Seed, Thomas M.; Sechelski, John (1978). "The biological effects of tryptophol (indole-3-ethanol): Hemolytic, biochemical and behavior modifying activity". Comparative Biochemistry and Physiology C. 60 (2): 175–185. doi:10.1016/0306-4492(78)90091-6.
- ↑ Gil, C.; Gómez-Cordovés, C. (1986). "Tryptophol content of young wines made from Tempranillo, Garnacha, Viura and Airén grapes". Food Chemistry. 22: 59–65. doi:10.1016/0308-8146(86)90009-9.
- ↑ Szlavko, Clara M. (1973). "Tryptophol, Tyrosol and Phenylethanol-The Aromatic Higher Alcohols in Beer". Journal of the Institute of Brewing. 79 (4): 283–288. doi:10.1002/j.2050-0416.1973.tb03541.x.
- ↑ Ribéreau-Gayon, P; Sapis, JC (1965). "On the presence in wine of tyrosol, tryptophol, phenylethyl alcohol and gamma-butyrolactone, secondary products of alcoholic fermentation". Comptes rendus hebdomadaires des seances de l'Academie des sciences. Serie D: Sciences naturelles. 261 (8): 1915–6. PMID 4954284. (Article in French)
- ↑ Lingappa, BT; Prasad, M; Lingappa, Y; Hunt, DF; Biemann, K (1969). "Phenethyl alcohol and tryptophol: Autoantibiotics produced by the fungus Candida albicans". Science. 163 (3863): 192–4. doi:10.1126/science.163.3863.192. PMID 5762768.
- ↑ ErdoĞAn i, I; Sener, B; Higa, T (2000). "Tryptophol, a plant auxin isolated from the marine sponge Ircinia spinulosa". Biochemical systematics and ecology. 28 (8): 793–794. doi:10.1016/S0305-1978(99)00111-8. PMID 10856636.
- ↑ Richard W. Jackson (1930). "A synthesis of tryptophol" (PDF). Journal of Biological Chemistry. 88 (3): 659–662.
- ↑ Dickinson, JR; Salgado, LE; Hewlins, MJ (2003). "The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae". The Journal of Biological Chemistry. 278 (10): 8028–34. doi:10.1074/jbc.M211914200. PMID 12499363.
- ↑ Pathway: tryptophan degradation VIII (to tryptophol) at BioCyc.org
- ↑ Stibbs, H. H.; Seed, J. R. (1975). "Short-Term Metabolism of \14C]Tryptophan in Rats Infected with Trypanosoma brucei gambiense". Journal of Infectious Diseases. 131 (4): 459–62. doi:10.1093/infdis/131.4.459. PMID 1117200.
- ↑ Toshiko Furukawa, Jinichiro Koga, Takashi Adachi, Kunihei Kishi and Kunihiko Syono (1996). "Efficient Conversion of L-Tryptophan to Indole-3-Acetic Acid and/or Tryptophol by Some Species of Rhizoctonia". Plant Cell Physiol. 37 (7): 899–905. doi:10.1093/oxfordjournals.pcp.a029037.
- ↑ Brown HM, Purves WK (1976). "Isolation and characterization of indole-3-acetaldehyde reductases from Cucumis sativus". J. Biol. Chem. 251 (4): 907–13. PMID 2607.
- ↑ Laćan, G; Magnus, V; Jericević, B; Kunst, L; Iskrić, S (1984). "Formation of Tryptophol Galactoside and an Unknown Tryptophol Ester in Euglena gracilis". Plant Physiology. 76 (4): 889–93. doi:10.1104/pp.76.4.889. PMC 1064400
 . PMID 16663965.
. PMID 16663965. - 1 2 Cornford, Eain M.; Crane, Paul D.; Braun, Leon D.; Bocash, William D.; Nyerges, Anthony M.; Oldendorf, William H. (1981). "Reduction in Brain Glucose Utilization Rate after Tryptophol (3-Indole Ethanol) Treatment". Journal of Neurochemistry. 36 (5): 1758–65. doi:10.1111/j.1471-4159.1981.tb00428.x. PMID 7241135.
- ↑ Feldstein, A.; Chang, F.H.; Kucharski, J.M. (1970). "Tryptophol, 5-hydroxytryptophol and 5-methoxytryptophol induced sleep in mice". Life Sciences. 9 (6): 323–9. doi:10.1016/0024-3205(70)90220-1. PMID 5444013.
- 1 2 Kosalec, Ivan; Ramić, Snježana; Jelić, Dubravko; Antolović, Roberto; Pepeljnjak, Stjepan; Kopjar, Nevenka (2011). "Assessment of Tryptophol Genotoxicity in Four Cell Lines in Vitro: A Pilot Study with Alkaline Comet Assay". Archives of Industrial Hygiene and Toxicology. 62. doi:10.2478/10004-1254-62-2011-2090.
- ↑ Wuster, Arthur; Babu, M. Madan (2010). "Transcriptional control of the quorum sensing response in yeast". Molecular BioSystems. 6 (1): 134–41. doi:10.1039/B913579K. PMID 20024075.
- ↑ Ackerman, S. B.; Seed, J. R. (1976). "The effects of tryptophol on immune responses and its implications toward trypanosome-induced immunosuppression". Experientia. 32 (5): 645–7. doi:10.1007/BF01990212. PMID 776647.
- ↑ Seed, John Richard; Sechelski, John (1977). "Tryptophol levels in mice injected with pharmacological doses of tryptophol, and the effect of pyrazole and ethanol on these levels". Life Sciences. 21 (11): 1603–10. doi:10.1016/0024-3205(77)90237-5. PMID 600013.
- ↑ Rayle, DL; Purves, WK (1967). "Isolation and Identification of Indole-3-Ethanol (Tryptophol) from Cucumber Seedlings". Plant Physiology. 42 (4): 520–524. doi:10.1104/pp.42.4.520. PMC 1086576
 . PMID 16656532.
. PMID 16656532. - ↑ Jelaska, Sibila; Magnus, Volker; Seretin, Mira; Lacan, Goran (1985). "Induction of embryogenic callus in Cucurbita pepo hypocotyl explants by indole-3-ethanol and its sugar conjugates". Physiologia Plantarum. 64 (2): 237–242. doi:10.1111/j.1399-3054.1985.tb02342.x.
- ↑ "Dialkyltryptamines via Tryptophol - [www.rhodium.ws]". Erowid.org. Retrieved 2013-08-18.