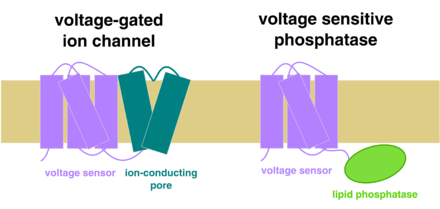
Voltage sensitive phosphatase
Voltage sensitive phosphatases or voltage sensor-containing phosphatases, commonly abbreviated VSPs, are a protein family found in many species, including humans, mice, zebrafish, frogs, and sea squirt.
| Identifiers | |
|---|---|
| Symbol | VSP |
| OPM superfamily | 8 |
| OPM protein | 4g80 |
Discovery
The first voltage sensitive phosphatase was discovered as a result of a genome-wide search in the sea squirt Ciona intestinalis.[1] The search was designed to identify proteins which contained a sequence of amino acids called a voltage sensor, because this sequence of amino acids confers voltage sensitivity to voltage-gated ion channels.[2][3][4] Although the initial genomic analysis was primarily concerned with the evolution of voltage-gated ion channels, one of the results of the work was the discovery of the VSP protein in sea squirt, termed Ci-VSP.[5]
The homologues to Ci-VSP in mammals are called Transmembrane phosphatases with tensin homology, or TPTEs. TPTE (now also called hVSP2[6]) and the closely related TPIP (also called TPTE2 or hVSP1[7]) were identified before the discovery of Ci-VSP,[8][9][10][11] however no voltage-dependent activity was described in the initial reports of these proteins. Subsequently, computational methods were used to suggest that these proteins may be voltage sensitive,[12] however Ci-VSP is still widely regarded as the first-identified VSP.[13][14]
Species and tissue distribution
VSPs are evolutionarily conserved from sea squirts up through humans. Most reports indicate that VSPs are found primarily in reproductive tissue, especially the testis.
Structure and Function
VSPs are made up of two protein domains: a voltage sensor domain, and a phosphatase domain.
The voltage sensor

The voltage sensor domain contains four transmembrane helices, named S1 through S4. The S4 transmembrane helix contains a number of positively charged arginine and lysine amino acid residues. Voltage sensitivity in VSPs is generated primarily by these charges in the S4, in much the same way that voltage-gated ion channels are gated by voltage. When positive charge builds up on one side of a membrane containing such voltage sensors, it generates an electric force pressing the S4 in the opposite direction. Changes in membrane potential therefore move the S4 back and forth through the membrane, allowing the voltage sensor to act like a switch. Activation of the voltage sensor occurs at depolarized potentials, i.e.: when the membrane collects more positive charge on the inner leaflet. Conversely, deactivation of the voltage sensor takes place at hyperpolarized potentials, when the membrane collects more negative charge on the inner leaflet. Activation of the voltage sensor increases the activity of the phosphatase domain, while deactivation of the voltage sensor decreases phosphatase activity.
The phosphatase
The phosphatase domain in VSPs is highly homologous to the tumor suppressor PTEN, and acts to remove phosphate groups from phospholipids in the membrane containing the VSP. Phospholipids such as inositol phosphates are signaling molecules which exert different effects depending on the pattern in which they are phosphorylated and dephosphorylated. Therefore, the action of VSPs is to indirectly regulate processes dependent on phospholipids.
The main substrate that has been characterized so far for VSPs (including hVSP1[15] but not hVSP2/TPTE, which shows no phosphatase activity) is phosphatidylinositol (4,5)-bisphosphate, which VSPs dephosphorylate at the 5' position.[16][17] However, VSP activity has been reported against other phosphoinositides as well, including phosphatidylinositol (3,4,5)-trisphosphate, which is also dephosphorylated at the 5' position.[18] Activity against the 3-phosphate of PI(3,4)P2 has also been demonstrated; this activity seems to become apparent at high membrane potentials, at lower potentials the 5'-phosphatase activity is predominant.[19]
X-Ray crystal structures
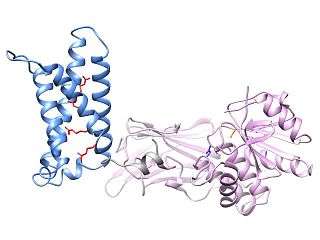
X-ray crystallography has been used to generate high-resolution images of the two domains of Ci-VSP, separate from one another.[20][21][22] By introducing small mutations in the protein, researchers have produced crystal structures of both the voltage sensing domain and the phosphatase domain from Ci-VSP in what are thought to be the "on" and "off" states. These structures have led to a model of VSP activation where movement of the voltage sensor affects a conformational change in a "gating loop," moving a glutamate residue in the gating loop away from the catalytic pocket of the phosphatase domain to increase phosphatase activity.
Uses in research and in biology
VSPs have been used as an tool to manipulate phospholipids in experimental settings. Because membrane potential can be controlled using patch clamp techniques, placing VSPs in a membrane allows for experimenters to rapidly dephosphorylate substrates of VSPs. VSPs' voltage sensors have also been used to engineer genetically encodable voltage sensitive fluorescent probes. These probes allow experimenters to visualize voltage in membranes using fluorescence. However, the normal role which VSPs play in the body is still not well understood.
See also
References
- ↑ Okamura, Y.; Nishino, A.; Murata, Y.; Nakajo, K.; Iwasaki, H.; Ohtsuka, Y.; Tanaka-Kunishima, M.; Takahashi, N.; Hara, Y.; Yoshida, T.; Nishida, M.; Okado, H.; Watari, H.; Meinertzhagen, I. A.; Satoh, N.; Takahashi, K.; Satou, Y.; Okada, Y.; Mori, Y. (2005). "Comprehensive analysis of the ascidian genome reveals novel insights into the molecular evolution of ion channel genes". Physiological Genomics. 22 (3): 269–282. doi:10.1152/physiolgenomics.00229.2004. PMID 15914577.
- ↑ Liman, E. R.; Hess, P.; Weaver, F.; Koren, G. (1991). "Voltage-sensing residues in the S4 region of a mammalian K+ channel". Nature. 353 (6346): 752–756. doi:10.1038/353752a0. PMID 1944534.
- ↑ Papazian, D. M.; Timpe, L. C.; Jan, Y. N.; Jan, L. Y. (1991). "Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence". Nature. 349 (6307): 305–310. doi:10.1038/349305a0. PMID 1846229.
- ↑ Shao, X. M.; Papazian, D. M. (1993). "S4 mutations alter the single-channel gating kinetics of Shaker K+ channels". Neuron. 11 (2): 343–352. doi:10.1016/0896-6273(93)90189-X. PMID 8352942.
- ↑ Murata, Y.; Iwasaki, H.; Sasaki, M.; Inaba, K.; Okamura, Y. (2005). "Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor". Nature. 435 (7046): 1239–1243. doi:10.1038/nature03650. PMID 15902207.
- ↑ Halaszovich, C. R.; Leitner, M. G.; Mavrantoni, A; Le, A; Frezza, L; Feuer, A; Schreiber, D. N.; Villalba-Galea, C. A.; Oliver, D (2012). "A human phospholipid phosphatase activated by a transmembrane control module". The Journal of Lipid Research. 53 (11): 2266–74. doi:10.1194/jlr.M026021. PMC 3465996
 . PMID 22896666.
. PMID 22896666. - ↑ Halaszovich, C. R.; Leitner, M. G.; Mavrantoni, A; Le, A; Frezza, L; Feuer, A; Schreiber, D. N.; Villalba-Galea, C. A.; Oliver, D (2012). "A human phospholipid phosphatase activated by a transmembrane control module". The Journal of Lipid Research. 53 (11): 2266–74. doi:10.1194/jlr.M026021. PMC 3465996
 . PMID 22896666.
. PMID 22896666. - ↑ Guipponi, M.; Tapparel, C.; Jousson, O.; Scamuffa, N.; Mas, C.; Rossier, C.; Hutter, P.; Meda, P.; Lyle, R.; Reymond, A.; Antonarakis, S. (2001). "The murine orthologue of the Golgi-localized TPTE protein provides clues to the evolutionary history of the human TPTE gene family". Human Genetics. 109 (6): 569–575. doi:10.1007/s004390100607. PMID 11810268.
- ↑ Walker, S. M.; Downes, C. P.; Leslie, N. R. (2001). "TPIP: A novel phosphoinositide 3-phosphatase". The Biochemical Journal. 360 (Pt 2): 277–283. doi:10.1042/0264-6021:3600277. PMC 1222227
 . PMID 11716755.
. PMID 11716755. - ↑ Wu, Y.; Dowbenko, D.; Pisabarro, M. T.; Dillard-Telm, L.; Koeppen, H.; Lasky, L. A. (2001). "PTEN 2, a Golgi-associated Testis-specific Homologue of the PTEN Tumor Suppressor Lipid Phosphatase". Journal of Biological Chemistry. 276 (24): 21745–21753. doi:10.1074/jbc.M101480200. PMID 11279206.
- ↑ Chen, H.; Rossier, C.; Morris, M. A.; Scott, H. S.; Gos, A.; Bairoch, A.; Antonarakis, S. E. (1999). "A testis-specific gene, TPTE, encodes a putative transmembrane tyrosine phosphatase and maps to the pericentromeric region of human chromosomes 21 and 13, and to chromosomes 15, 22, and Y". Human Genetics. 105 (5): 399–409. doi:10.1007/s004390051122. PMID 10598804.
- ↑ Kumanovics, A.; Levin, G.; Blount, P. (2002). "Family ties of gated pores: Evolution of the sensor module". The FASEB Journal. 16 (12): 1623–1629. doi:10.1096/fj.02-0238hyp. PMID 12374785.
- ↑ Okamura, Y.; Murata, Y.; Iwasaki, H. (2008). "Voltage-sensing phosphatase: Actions and potentials". The Journal of Physiology. 587 (3): 513–520. doi:10.1113/jphysiol.2008.163097. PMC 2670076
 . PMID 19074969.
. PMID 19074969. - ↑ Villalba-Galea, C. A. (2012). "Voltage-Controlled Enzymes: The New JanusBifrons". Frontiers in Pharmacology. 3: 161–110. doi:10.3389/fphar.2012.00161. PMC 3440755
 . PMID 22993507.
. PMID 22993507. - ↑ Halaszovich, C. R.; Leitner, M. G.; Mavrantoni, A; Le, A; Frezza, L; Feuer, A; Schreiber, D. N.; Villalba-Galea, C. A.; Oliver, D (2012). "A human phospholipid phosphatase activated by a transmembrane control module". The Journal of Lipid Research. 53 (11): 2266–74. doi:10.1194/jlr.M026021. PMC 3465996
 . PMID 22896666.
. PMID 22896666. - ↑ Iwasaki, H; Murata, Y; Kim, Y; Hossain, M. I.; Worby, C. A.; Dixon, J. E.; McCormack, T; Sasaki, T; Okamura, Y (2008). "A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate". Proceedings of the National Academy of Sciences. 105 (23): 7970–5. doi:10.1073/pnas.0803936105. PMC 2430346
 . PMID 18524949.
. PMID 18524949. - ↑ Halaszovich, C. R.; Schreiber, D. N.; Oliver, D (2009). "Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5'-phosphatase". Journal of Biological Chemistry. 284 (4): 2106–13. doi:10.1074/jbc.M803543200. PMID 19047057.
- ↑ Halaszovich, C. R.; Schreiber, D. N.; Oliver, D (2009). "Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5'-phosphatase". Journal of Biological Chemistry. 284 (4): 2106–13. doi:10.1074/jbc.M803543200. PMID 19047057.
- ↑ Kurokawa, T; Takasuga, S; Sakata, S; Yamaguchi, S; Horie, S; Homma, K. J.; Sasaki, T; Okamura, Y (2012). "3' Phosphatase activity toward phosphatidylinositol 3,4-bisphosphate PI(3,4)P2 by voltage-sensing phosphatase (VSP)". Proceedings of the National Academy of Sciences. 109 (25): 10089–94. doi:10.1073/pnas.1203799109. PMC 3382541
 . PMID 22645351.
. PMID 22645351. - ↑ Matsuda, M; Takeshita, K; Kurokawa, T; Sakata, S; Suzuki, M; Yamashita, E; Okamura, Y; Nakagawa, A (2011). "Crystal structure of the cytoplasmic phosphatase and tensin homolog (PTEN)-like region of Ciona intestinalis voltage-sensing phosphatase provides insight into substrate specificity and redox regulation of the phosphoinositide phosphatase activity". Journal of Biological Chemistry. 286 (26): 23368–77. doi:10.1074/jbc.M110.214361. PMC 3123101
 . PMID 21543329.
. PMID 21543329. - ↑ Li, Q; Wanderling, S; Paduch, M; Medovoy, D; Singharoy, A; McGreevy, R; Villalba-Galea, C. A.; Hulse, R. E.; Roux, B; Schulten, K; Kossiakoff, A; Perozo, E (2014). "Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain". Nature Structural & Molecular Biology. 21 (3): 244–52. doi:10.1038/nsmb.2768. PMID 24487958.
- ↑ Liu, L; Kohout, S. C.; Xu, Q; Müller, S; Kimberlin, C. R.; Isacoff, E. Y.; Minor Jr, D. L. (2012). "A glutamate switch controls voltage-sensitive phosphatase function". Nature Structural & Molecular Biology. 19 (6): 633–41. doi:10.1038/nsmb.2289. PMC 3529583
 . PMID 22562138.
. PMID 22562138.