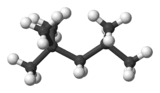
2,2,4-Trimethylpentane
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,2,4-Trimethylpentane[1] | |
| Identifiers | |
| 540-84-1 | |
| 3D model (Jmol) | Interactive image |
| 1696876 | |
| ChEBI | CHEBI:62805 |
| ChEMBL | ChEMBL1797261 |
| ChemSpider | 10445 |
| ECHA InfoCard | 100.007.964 |
| EC Number | 208-759-1 |
| MeSH | 2,2,4-trimethylpentane |
| PubChem | 10907 |
| RTECS number | SA3320000 |
| UN number | 1262 |
| |
| |
| Properties | |
| C8H18 | |
| Molar mass | 114.23 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 0.692 g cm−3 |
| Melting point | −107.38 °C; −161.28 °F; 165.77 K |
| Boiling point | 99.30 °C; 210.74 °F; 372.45 K |
| log P | 4.373 |
| Vapor pressure | 5.5 kPa (at 21 °C) |
| Henry's law constant (kH) |
3.0 nmol Pa−1 kg−1 |
| UV-vis (λmax) | 210 nm |
| Refractive index (nD) |
1.391 |
| Thermochemistry | |
| 242.49 J K−1 mol−1 | |
| Std molar entropy (S |
328.03 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
−260.6 to −258.0 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−5462.6 to −5460.0 kJ mol−1 |
| Hazards | |
| GHS pictograms |     |
| GHS signal word | DANGER |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+310, P331 | |
| EU classification (DSD) |
|
| R-phrases | R11, R38, R50/53, R65, R67 |
| S-phrases | (S2), S16, S29, S33 |
| NFPA 704 | |
| Flash point | −12 °C (10 °F; 261 K) |
| 396 °C (745 °F; 669 K) | |
| Explosive limits | 1.1–6.0% |
| Related compounds | |
| Related alkanes |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale (the zero point is n-heptane). It is an important component of gasoline, frequently used in relatively large proportions to increase the knock resistance of the fuel.[2]
Strictly speaking, if the standard meaning of ‘iso’ is followed, the name isooctane should be reserved for the isomer 2-methylheptane. However, 2,2,4-trimethylpentane is by far the most important isomer of octane and so, historically, it has ended up with this name.[3]
Production
Isooctane is produced on a massive scale in the petroleum industry by distillation of petroleum. It can also be produced from isobutylene by dimerization (a variant of alkylation) using an Amberlyst catalyst to produce a mixture of iso-octenes. Hydrogenation of this mixture produces 2,2,4-trimethylpentane.[4]
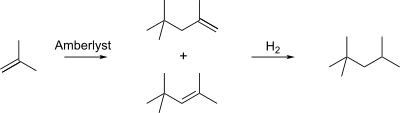 Dimerization of isobutylene followed by hydrogenation produces 2,2,4-trimethylpentane
Dimerization of isobutylene followed by hydrogenation produces 2,2,4-trimethylpentane
History
Engine knocking is an unwanted process that can occur during combustion in internal combustion engines. Graham Edgar in 1926 added different amounts of n-heptane and 2,2,4-trimethylpentane to gasoline, and discovered that the knocking stopped when 2,2,4-trimethylpentane was added. This was the origin of the octane rating scale.[5] Test motors using 2,2,4-trimethylpentane gave a certain performance which was standardized as 100 octane. The same test motors, run in the same fashion, using heptane, gave a performance which was standardized as 0 octane. All other compounds and blends of compounds then were graded against these two standards and assigned octane numbers.
Safety
In common with all hydrocarbons, 2,2,4-trimethylpentane is exceedingly flammable in the presence of oxygen, and its vapours readily form explosive mixtures with air.
Also in common with all hydrocarbons, inhalation or ingestion of large quantities of 2,2,4-trimethylpentane is harmful. In rare cases a stronger reaction can occur.[6]
See also
References
- ↑ "2,2,4-trimethylpentane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 11 March 2012.
- ↑ Werner Dabelstein, Arno Reglitzky, Andrea Schütze and Klaus Reders "Automotive Fuels" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim.doi:10.1002/14356007.a16_719.pub2
- ↑ Clayden, Jonathan (2005). Organic chemistry (Reprinted (with corrections). ed.). Oxford [u.a.]: Oxford Univ. Press. p. 315. ISBN 978-0-19-850346-0.
- ↑ Dimerization of isobutylene, Amberlyst.com
- ↑ Fuels and lubricants handbook, Volume 1, George E. Totten, Steven R. Westbrook, Rajesh J. Shah, page 62
- ↑ 2,2,4-Trimethylpentane, Integrated Risk Information System, United States Environmental Protection Agency
