Adenocarcinoma of the lung
Adenocarcinoma of the lung (pulmonary adenocarcinoma) is a common histological form of lung cancer that contains certain distinct malignant tissue architectural, cytological, or molecular features, including gland and/or duct formation and/or production of significant amounts of mucus.[1]
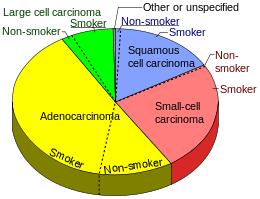
Epidemiology
Nearly 40% of lung cancers in the US are adenocarcinoma, which usually originates in peripheral lung tissue.[3] Most cases of adenocarcinoma are associated with smoking; however, among people who have smoked fewer than 100 cigarettes in their lifetimes ("never-smokers"),[4] adenocarcinoma is the most common form of lung cancer.[5] Its incidence has been increasing in many developed Western nations in the past few decades, where it has become the most common major type of lung cancer in smokers (replacing squamous cell lung carcinoma) and in lifelong nonsmokers.[1] According to the Nurses' Health Study, the risk of adenocarcinoma of the lung increases substantially after a long duration of previous tobacco smoking, with a previous smoking duration of 30 to 40 years giving a relative risk of approximately 2.4 compared to never-smokers, and a duration of more than 40 years giving a relative risk of approximately 5.[6]
This cancer usually is seen peripherally in the lungs, as opposed to small cell lung cancer and squamous cell lung cancer, which both tend to be more centrally located,[7][8] although it may also occur as central lesions.[8] For unknown reasons, it often arises in relation to peripheral lung scars. The current theory is that the scar probably occurred secondary to the tumor, rather than causing the tumor.[8] The adenocarcinoma has an increased incidence in smokers, and is the most common type of lung cancer seen in non-smokers and women.[8] The peripheral location of adenocarcinoma in the lungs may be due to the use of filters in cigarettes which prevent the larger particles from entering the lung.[9][10] Deeper inhalation of cigarette smoke results in peripheral lesions that are often the case in adenocarcinomas of the lung. Generally, adenocarcinomas grow more slowly and form smaller masses than the other subtypes.[8] However, they tend to form metastases widely at an early stage.[8] Adenocarcinoma is a non-small cell lung carcinoma, and as such, it is not as responsive to radiation therapy as is small cell lung carcinoma, but is rather treated surgically, for example by pneumonectomy or lobectomy.[8]
Classification

Adenocarcinomas are highly heterogeneous tumors. Several major histological subtypes are currently recognized by the WHO [1] and IASLC/ATS/ERS[11][12][13]
- Non-invasive or minimally invasive adenocarcinoma
- Invasive adenocarcinoma
- Acinar predominant adenocarcinoma
- Papillary predominant adenocarcinoma
- Micropapillary predominant adenocarcinoma
- Solid predominant adenocarcinoma
- Invasive mucinous adenocarcinoma
In as many as 80% of tumors that are extensively sampled, components of more than one of these subtypes will be recognized. In such cases, resected tumors should be classified by comprehensive histological subtyping. Using increments of 5% to describe the amount of each subtype present, the predominant subtype is used to classify the whole tumor.[14] The predominant subtype is prognostic for survival after complete resection.[15]
Signet ring and clear cell adenocarcinoma are no longer histological subtypes, but rather cytological features that can occur in tumour cells of multiple histological subtypes, most often solid adenocarcinoma.[11]
Some variants are not clearly recognized by the WHO and IASLC/ATS/ERS classification:
Histopathology
Adenocarcinoma of the lung tends to stain mucin positive as it is derived from the mucus producing glands of the lungs. Similar to other adenocarcinoma, if this tumor is well differentiated (low grade) it will resemble the normal glandular structure. Poorly differentiated adenocarcinoma will not resemble the normal glands (high grade) and will be detected by seeing that they stain positive for mucin (which the glands produce).[18][19] Adenocarcinoma can also be distinguished by staining for TTF-1, a cell marker for adenocarcinoma.[3]
To reveal the adenocarcinomatous lineage of the solid variant, demonstration of intracellular mucin production may be performed. Foci of squamous metaplasia and dysplasia may be present in the epithelium proximal to adenocarcinomas, but these are not the precursor lesions for this tumor. Rather, the precursor of peripheral adenocarcinomas has been termed atypical adenomatous hyperplasia (AAH).[8] Microscopically, AAH is a well-demarcated focus of epithelial proliferation, containing cuboidal to low-columnar cells resembling club cells or type II pneumocytes.[8] These demonstrate various degrees of cytologic atypia, including hyperchromasia, pleomorphism, prominent nucleoli.[8] However, the atypia is not to the extent as seen in frank adenocarcinomas.[8] Lesions of AAH are monoclonal, and they share many of the molecular aberrations (like KRAS mutations) that are associated with adenocarcinomas.[8]
Management
Targeted therapy is available for lung adenocarcinomas with certain mutations. Crizotinib is effective in tumors with fusions involving ALK or ROS1, whereas gefitinib, erlotinib, and afatinib are used in patients whose tumors have mutations in EGFR.[3]
Molecular biology
Chromosomal rearrangements
Three membrane associated tyrosine kinase receptors are recurrently involved in rearrangements in adenocarcinomas: ALK, ROS1, and RET, and more than eighty other translocations have also been reported in adenocarcinomas of the lung.[20] Targeted therapies: ALK and ROS1 fusions proteins are both sensitive to treatment with the new ALK tyrosine kinase inhibitors (see the Atlas of Genetics and Cytogenetics in Oncology and Haematology,[21]).
Gene mutations
Common gene mutations in pulmonary adenocarcinoma affect many genes, including EGFR (20%), HER2 (2%), KRAS, ALK, BRAF, PIK3CA, MET (1%, associated with resistant disease), and ROS1. Most of these genes are kinases, and can be mutated in different ways, including amplification.[3]
References
- 1 2 3 Travis, William D; Brambilla, Elisabeth; Müller-Hermelink, H Konrad; Harris, Curtis C, eds. (2004). Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart (PDF). World Health Organization Classification of Tumours. Lyon: IARC Press. ISBN 92-832-2418-3. Retrieved 27 March 2010.
- ↑ Smokers defined as current or former smoker of more than 1 year of duration. See image page in Commons for percentages in numbers. Reference:
- Table 2 in: Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA (2008). "Comparison of aspects of smoking among the four histological types of lung cancer.". Tob Control. 17 (3): 198–204. doi:10.1136/tc.2007.022582. PMC 3044470
 . PMID 18390646.
. PMID 18390646.
- Table 2 in: Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA (2008). "Comparison of aspects of smoking among the four histological types of lung cancer.". Tob Control. 17 (3): 198–204. doi:10.1136/tc.2007.022582. PMC 3044470
- 1 2 3 4 World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.1. ISBN 9283204298.
- ↑ Horn, L; Pao W; Johnson DH (2012). "Chapter 89". In Longo, DL; Kasper, DL; Jameson, JL; Fauci, AS; Hauser, SL; Loscalzo, J. Harrison's Principles of Internal Medicine (18th ed.). McGraw-Hill. ISBN 0-07-174889-X.
- ↑ Subramanian, J; Govindan R (February 2007). "Lung cancer in never smokers: a review". Journal of Clinical Oncology. American Society of Clinical Oncology. 25 (5): 561–570. doi:10.1200/JCO.2006.06.8015. PMID 17290066.
- ↑ Kenfield, S. A.; Wei, E. K.; Stampfer, M. J.; Rosner, B. A.; Colditz, G. A. (2008). "Comparison of aspects of smoking among the four histological types of lung cancer". Tobacco Control. 17 (3): 198–204. doi:10.1136/tc.2007.022582. PMC 3044470
 . PMID 18390646.
. PMID 18390646. - ↑ Travis WD, Travis LB, Devesa SS (January 1995). "Lung cancer". Cancer. 75 (1 Suppl): 191–202. doi:10.1002/1097-0142(19950101)75:1+<191::AID-CNCR2820751307>3.0.CO;2-Y. PMID 8000996.
- 1 2 3 4 5 6 7 8 9 10 11 12 Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson. "Chapter 13, box on morphology of adenocarcinoma". Robbins Basic Pathology (8th ed.). Philadelphia: Saunders. ISBN 1-4160-2973-7.
- ↑ Goljan USMLE Audio Tapes, 2001
- ↑ British Journal of Cancer (2004) 90, 646 – 651 T Marugame et al, Filter cigarette smoking and lung cancer risk; a hospital-based case–control study in Japan
- 1 2 Van Schil, P. E.; Asamura, H; Rusch, V. W.; Mitsudomi, T; Tsuboi, M; Brambilla, E; Travis, W. D. (2012). "Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification". European Respiratory Journal. 39 (2): 478–86. doi:10.1183/09031936.00027511. PMID 21828029.
- ↑ Travis, W. D.; Brambilla, E; Van Schil, P; Scagliotti, G. V.; Huber, R. M.; Sculier, J. P.; Vansteenkiste, J; Nicholson, A. G. (2011). "Paradigm shifts in lung cancer as defined in the new IASLC/ATS/ERS lung adenocarcinoma classification". European Respiratory Journal. 38 (2): 239–43. doi:10.1183/09031936.00026711. PMID 21804158.
- ↑ Vazquez, M; Carter, D; Brambilla, E; Gazdar, A; Noguchi, M; Travis, W. D.; Huang, Y; Zhang, L; Yip, R; Yankelevitz, D. F.; Henschke, C. I.; International Early Lung Cancer Action Program Investigators (2009). "Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: Histopathologic features and their prognostic implications". Lung Cancer. 64 (2): 148–54. doi:10.1016/j.lungcan.2008.08.009. PMC 2849638
 . PMID 18951650.
. PMID 18951650. - ↑ Travis, W. D.; Brambilla, E; Noguchi, M; Nicholson, A. G.; Geisinger, K. R.; Yatabe, Y; Beer, D. G.; Powell, C. A.; Riely, G. J.; Van Schil, P. E.; Garg, K; Austin, J. H.; Asamura, H; Rusch, V. W.; Hirsch, F. R.; Scagliotti, G; Mitsudomi, T; Huber, R. M.; Ishikawa, Y; Jett, J; Sanchez-Cespedes, M; Sculier, J. P.; Takahashi, T; Tsuboi, M; Vansteenkiste, J; Wistuba, I; Yang, P. C.; Aberle, D; Brambilla, C; et al. (2011). "International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma". Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 6 (2): 244–85. doi:10.1097/JTO.0b013e318206a221. PMC 4513953
 . PMID 21252716.
. PMID 21252716. - ↑ Russell, P. A.; Wainer, Z; Wright, G. M.; Daniels, M; Conron, M; Williams, R. A. (2011). "Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification". Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 6 (9): 1496–504. doi:10.1097/JTO.0b013e318221f701. PMID 21642859.
- ↑ Yousem, S. A. (2005). "Pulmonary intestinal-type adenocarcinoma does not show enteric differentiation by immunohistochemical study". Modern Pathology. 18 (6): 816–21. doi:10.1038/modpathol.3800358. PMID 15605076.
- ↑ Lin, D; Zhao, Y; Li, H; Xing, X (2013). "Pulmonary enteric adenocarcinoma with villin brush border immunoreactivity: A case report and literature review". Journal of thoracic disease. 5 (1): E17–20. doi:10.3978/j.issn.2072-1439.2012.06.06. PMC 3547996
 . PMID 23372961.
. PMID 23372961. - ↑ Diseases of Lung
- ↑ Adenocarcinoma of Lung (Mucin Stain)
- ↑ http://atlasgeneticsoncology.org/Tumors/TranslocLungAdenocarcID6751.html
- ↑ "Atlas of Genetics and Cytogenetics in Oncology and Haematology". atlasgeneticsoncology.org.