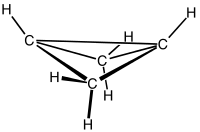
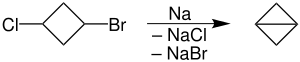Bicyclobutane
 | |
| Identifiers | |
|---|---|
| 157-33-5 | |
| Properties | |
| C4H6 | |
| Appearance | colorless gas |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bicyclobutane is an organic compound with the formula C4H6. Consisting of two fused cyclopropane rings, it is one of the most strained compounds isolatable on a large scale. It is a colorless easily condensed gas.[1]
The first reported bicyclobutane was the carboxyethyl derivative, C4H5CO2Et, which was prepared by dehydrohalogenation the corresponding bromocyclobutanecarboxylate ester. The parent hydrocarbon was prepared by conversion of bromocyclobutanecarboxylate ester to 1-bromo-3-chlorocyclobutane, followed intramolecular Wurtz coupling using molten sodium:[2]
It is a nonplanar molecule, with a dihedral angle of 123°.
References
- ↑ K. B. Wiberg, G. M. Lampman, R. P. Ciula, D. S. Connor,P. Schertler, J. Lavanish "Bicyclo[1.1.0]butane" Tetrahedron, 1965. Vol. 11, pp. 2749 - 2769.
- ↑ Gary M. Lampman and James C. Aumiller "Bicyclo[1.1.0]butane" Organic Syntheses 1971, volume 51, 55..doi:10.15227/orgsyn.051.0055
This article is issued from Wikipedia - version of the 8/23/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.