Big Bang nucleosynthesis
| Part of a series on | |||
| Physical cosmology | |||
|---|---|---|---|
 | |||
|
Early universe
|
|||
|
Components · Structure |
|||
| |||
In physical cosmology, Big Bang nucleosynthesis (abbreviated BBN, also known as primordial nucleosynthesis, arch(a)eonucleosynthesis, archonucleosynthesis, protonucleosynthesis and pal(a)eonucleosynthesis) refers to the production of nuclei other than those of the lightest isotope of hydrogen (hydrogen-1, 1H, having a single proton as a nucleus) during the early phases of the universe. Primordial nucleosynthesis is believed by most cosmologists to have taken place from 10 seconds to 20 minutes after the Big Bang, and is calculated to be responsible for the formation of most of the universe's helium as the isotope helium-4 (4He), along with small amounts of the hydrogen isotope deuterium (2H or D), the helium isotope helium-3 (3He), and a very small amount of the lithium isotope lithium-7 (7Li). In addition to these stable nuclei, two unstable or radioactive isotopes were also produced: the heavy hydrogen isotope tritium (3H or T); and the beryllium isotope beryllium-7 (7Be); but these unstable isotopes later decayed into 3He and 7Li, as above.
Essentially all of the elements that are heavier than lithium were created much later, by stellar nucleosynthesis in evolving and exploding stars.
Characteristics
There are two important characteristics of Big Bang nucleosynthesis (BBN):
- The era began at temperatures of around 10 MeV (116 gigakelvin) and ended at temperatures below 100 keV (1.16 gigakelvin).[1] The corresponding time interval was from a few tenths of a second to up to 103 seconds.[2] The temperature/time relation in this era can be given by the equation:
- ,
- where t is time in seconds, T is temperature in MeV and g* is the effective number of particle species.[3] (g* includes contributions of 2 from photons, 7/2 from electron-positron pairs and 7/4 from each neutrino flavor. In the standard model g* is 10.75). This expression also shows how a different number of neutrino flavors will change the rate of cooling of the early universe.
- It was widespread, encompassing the entire observable universe.
The key parameter which allows one to calculate the effects of BBN is the baryon/photon number ratio, which is a small number of order 6 x 10−10. This parameter corresponds to the baryon density and controls the rate at which nucleons collide and react; from this we can derive elemental abundances. Although the baryon per photon ratio is important in determining elemental abundances, the precise value makes little difference to the overall picture. Without major changes to the Big Bang theory itself, BBN will result in mass abundances of about 75% of hydrogen-1, about 25% helium-4, about 0.01% of deuterium and helium-3, trace amounts (on the order of 10−10) of lithium, and negligible heavier elements. (Traces of boron have been found in some old stars, giving rise to the question whether some boron, not really predicted by the theory, might have been produced in the Big Bang. The question is not presently resolved.[4]) That the observed abundances in the universe are generally consistent with these abundance numbers is considered strong evidence for the Big Bang theory.
In this field, for historical reasons it is customary to quote the helium-4 fraction by mass, symbol Y, so that 25% helium-4 means that helium-4 atoms account for 25% of the mass, but less than 8% of the nuclei would be helium-4 nuclei. Other (trace) nuclei are usually expressed as number ratios to hydrogen.
Important parameters
The creation of light elements during BBN was dependent on a number of parameters; among those was the neutron-proton ratio (calculable from Standard Model physics) and the baryon-photon ratio.
Neutron–proton ratio
Neutrons can react with positrons or electron neutrinos to create protons and other products in one of the following reactions:
- n + e+ ↔ anti-νe + p
- n + νe ↔ p + e−
At times much earlier than 1 sec, the n/p ratio was close to 1:1. As the temperature dropped, the equilibrium shifted in favour of protons due to their slightly lower mass, and the n/p ratio decreased. These reactions continue until expansion of the universe outpaces the reactions, which occurs at about T = 0.7 MeV and is called the freeze out temperature. At freeze out, the neutron-proton ratio was about 1/6. However, free neutrons are unstable with a mean life of 880 sec; some neutrons decayed in the next few minutes before fusing into any nucleus, so the ratio of total neutrons to protons after nucleosynthesis ends is about 1/7. Almost all neutrons that fused instead of decaying ended up combined into helium-4, due to the fact that helium-4 has the highest binding energy per nucleon among light elements. This predicts that about 8% of all atoms should be helium-4, leading to a mass fraction of helium-4 of about 25%, which is in line with observations. Small traces of deuterium and helium-3 remained as there was insufficient time and density for them to react and form helium-4.[5]
Baryon–photon ratio
The baryon–photon ratio, η, is the key parameter determining the abundance of light elements present in the early universe. Baryons can react with light elements in the following reactions:
- (p,n) + 2H → (3He, 3H)
- (3He, 3H) + (n,p) → 4He
It is evident that reactions with baryons during BBN would ultimately result in helium-4, and also that the abundance of primordial deuterium is indirectly related to the baryon density or baryon-photon ratio. That is, the larger the baryon-photon ratio the more reactions there will be and the more efficiently deuterium will be eventually transformed into helium-4. This result makes deuterium a very useful tool in measuring the baryon-to-photon ratio.
Sequence
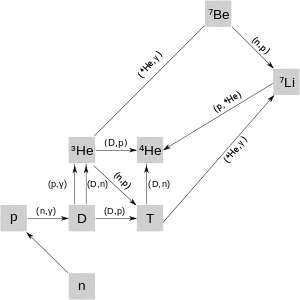
Big Bang nucleosynthesis began a few seconds after the big bang, when the universe had cooled sufficiently to allow deuterium nuclei to survive disruption by high-energy photons. This time is essentially independent of dark matter content, since the universe was highly radiation dominated until much later, and this dominant component controls the temperature/time relation. The relative abundances of protons and neutrons follow from simple thermodynamical arguments, combined with the way that the mean temperature of the universe changes over time. If the reactions needed to reach the thermodynamically favoured equilibrium values are too slow compared to the temperature change brought about by the expansion, abundances would have remained at some specific non-equilibrium value. Combining thermodynamics and the changes brought about by cosmic expansion, one can calculate the fraction of protons and neutrons based on the temperature at this point. The answer is that there are about seven protons for every neutron at the beginning of nucleosynthesis. This fraction is in favour of protons, primarily because their lower mass - with respect to the neutron - favors their production. Free neutrons decay to protons with a half-life of about 10.2 minutes, but this time-scale is longer than the first three minutes of nucleogenesis. It was during this time that a substantial fraction of neutrons were combined with protons into deuterium and then into He-4. The sequence of these reaction chains is shown on the image.[6]
One feature of BBN is that the physical laws and constants that govern the behavior of matter at these energies are very well understood, and hence BBN lacks some of the speculative uncertainties that characterize earlier periods in the life of the universe. Another feature is that the process of nucleosynthesis is determined by conditions at the start of this phase of the life of the universe, and proceeds independently of what happened before.
As the universe expands, it cools. Free neutrons and protons are less stable than helium nuclei, and the protons and neutrons have a strong tendency to form helium-4. However, forming helium-4 requires the intermediate step of forming deuterium. Before nucleosynthesis began, the temperature was high enough for many photons to have energy greater than the binding energy of deuterium; therefore any deuterium that was formed was immediately destroyed (a situation known as the deuterium bottleneck). Hence, the formation of helium-4 is delayed until the universe became cool enough for deuterium to survive (at about T = 0.1 MeV); after which there was a sudden burst of element formation. However, very shortly thereafter, at twenty minutes after the Big Bang, the universe became too cool for any further nuclear fusion and nucleosynthesis to occur. At this point, the elemental abundances were nearly fixed, and only change was the result of the radioactive decay of the two major unstable products of BBN, tritium and beryllium-7.[7]
History of theory
The history of Big Bang nucleosynthesis began with the calculations of Ralph Alpher in the 1940s. Alpher published the Alpher–Bethe–Gamow paper that outlined the theory of light-element production in the early universe.
During the 1970s, there was a major puzzle in that the density of baryons as calculated by Big Bang nucleosynthesis was much less than the observed mass of the universe based on calculations of the expansion rate. This puzzle was resolved in large part by postulating the existence of dark matter.
Heavy elements
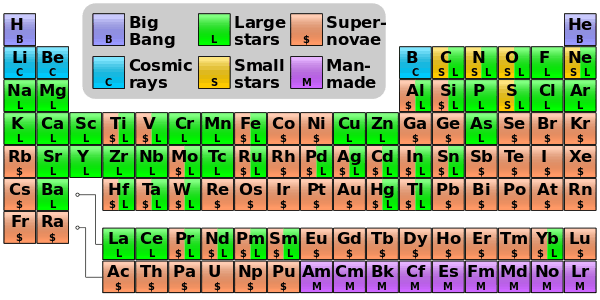
Big Bang nucleosynthesis produced no elements heavier than lithium, due to a bottleneck: the absence of a stable nucleus with 8 or 5 nucleons. This deficit of larger atoms also limited the amounts of lithium-7 produced during BBN. In stars, the bottleneck is passed by triple collisions of helium-4 nuclei, producing carbon (the triple-alpha process). However, this process is very slow, taking tens of thousands of years to convert a significant amount of helium to carbon in stars, and therefore it made a negligible contribution in the minutes following the Big Bang.
In proton-rich regions of the early universe, lithium-6, beryllium-9, and boron-11 could have been produced in the reactions 4He(D,γ)6Li, 7Li(T,n)9Be, 7Li(D,γ)9Be, 7Be(T,p)9Be, and 9Be(T,n)11B.[8][9] Carbon-12 can then be produced when boron-11 captures a neutron or deuteron, allowing for the formation of nitrogen and oxygen in the CNO cycle. The predicted abundance of CNO isotopes produced in Big Bang nucleosynthesis is expected to be on the order of 10−15 that of H, making them undetectable and negligible.[8] Indeed, none of these primordial isotopes of the elements from lithium to oxygen have yet been detected, although those of beryllium and boron may be able to be detected in the future. So far, the only stable nuclides known experimentally to have been made before or during Big Bang nucleosynthesis are protium, deuterium, helium-3, helium-4, and lithium-7.[10]
Helium-4
Big Bang nucleosynthesis predicts a primordial abundance of about 25% helium-4 by mass, irrespective of the initial conditions of the universe. As long as the universe was hot enough for protons and neutrons to transform into each other easily, their ratio, determined solely by their relative masses, was about 1 neutron to 7 protons (allowing for some decay of neutrons into protons). Once it was cool enough, the neutrons quickly bound with an equal number of protons to form first deuterium, then helium-4. Helium-4 is very stable and is nearly the end of this chain if it runs for only a short time, since helium neither decays nor combines easily to form heavier nuclei (since there are no stable nuclei with mass numbers of 5 or 8, helium does not combine easily with either protons, or with itself). Once temperatures are lowered, out of every 16 nucleons (2 neutrons and 14 protons), 4 of these (25% of the total particles and total mass) combine quickly into one helium-4 nucleus. This produces one helium for every 12 hydrogens, resulting in a universe that is a little over 8% helium by number of atoms, and 25% helium by mass.
One analogy is to think of helium-4 as ash, and the amount of ash that one forms when one completely burns a piece of wood is insensitive to how one burns it. The resort to the BBN theory of the helium-4 abundance is necessary as there is far more helium-4 in the universe than can be explained by stellar nucleosynthesis. In addition, it provides an important test for the Big Bang theory. If the observed helium abundance is much different from 25%, then this would pose a serious challenge to the theory. This would particularly be the case if the early helium-4 abundance was much smaller than 25% because it is hard to destroy helium-4. For a few years during the mid-1990s, observations suggested that this might be the case, causing astrophysicists to talk about a Big Bang nucleosynthetic crisis, but further observations were consistent with the Big Bang theory.[11]
Deuterium
Deuterium is in some ways the opposite of helium-4 in that while helium-4 is very stable and very difficult to destroy, deuterium is only marginally stable and easy to destroy. The temperatures, time, and densities were sufficient to combine a substantial fraction of the deuterium nuclei to form helium-4 but insufficient to carry the process further using helium-4 in the next fusion step. BBN did not convert all of the deuterium in the universe to helium-4 due to the expansion that cooled the universe and reduced the density and so, cut that conversion short before it could proceed any further. One consequence of this is that unlike helium-4, the amount of deuterium is very sensitive to initial conditions. The denser the initial universe was, the more deuterium would be converted to helium-4 before time ran out, and the less deuterium would remain.
There are no known post-Big Bang processes which can produce significant amounts of deuterium. Hence observations about deuterium abundance suggest that the universe is not infinitely old, which is in accordance with the Big Bang theory.
During the 1970s, there were major efforts to find processes that could produce deuterium, but those revealed ways of producing isotopes other than deuterium. The problem was that while the concentration of deuterium in the universe is consistent with the Big Bang model as a whole, it is too high to be consistent with a model that presumes that most of the universe is composed of protons and neutrons. If one assumes that all of the universe consists of protons and neutrons, the density of the universe is such that much of the currently observed deuterium would have been burned into helium-4. The standard explanation now used for the abundance of deuterium is that the universe does not consist mostly of baryons, but that non-baryonic matter (also known as dark matter) makes up most of the mass of the universe. This explanation is also consistent with calculations that show that a universe made mostly of protons and neutrons would be far more clumpy than is observed.
It is very hard to come up with another process that would produce deuterium other than by nuclear fusion. Such a process would require that the temperature be hot enough to produce deuterium, but not hot enough to produce helium-4, and that this process should immediately cool to non-nuclear temperatures after no more than a few minutes. It would also be necessary for the deuterium to be swept away before it reoccurs.
Producing deuterium by fission is also difficult. The problem here again is that deuterium is very unlikely due to nuclear processes, and that collisions between atomic nuclei are likely to result either in the fusion of the nuclei, or in the release of free neutrons or alpha particles. During the 1970s, cosmic ray spallation was proposed as a source of deuterium. That theory failed to account for the abundance of deuterium, but led to explanations of the source of other light elements.
Measurements and status of theory
The theory of BBN gives a detailed mathematical description of the production of the light "elements" deuterium, helium-3, helium-4, and lithium-7. Specifically, the theory yields precise quantitative predictions for the mixture of these elements, that is, the primordial abundances at the end of the big-bang.
In order to test these predictions, it is necessary to reconstruct the primordial abundances as faithfully as possible, for instance by observing astronomical objects in which very little stellar nucleosynthesis has taken place (such as certain dwarf galaxies) or by observing objects that are very far away, and thus can be seen in a very early stage of their evolution (such as distant quasars).
As noted above, in the standard picture of BBN, all of the light element abundances depend on the amount of ordinary matter (baryons) relative to radiation (photons). Since the universe is presumed to be homogeneous, it has one unique value of the baryon-to-photon ratio. For a long time, this meant that to test BBN theory against observations one had to ask: can all of the light element observations be explained with a single value of the baryon-to-photon ratio? Or more precisely, allowing for the finite precision of both the predictions and the observations, one asks: is there some range of baryon-to-photon values which can account for all of the observations?
More recently, the question has changed: Precision observations of the cosmic microwave background radiation[12][13] with the Wilkinson Microwave Anisotropy Probe (WMAP) and Planck give an independent value for the baryon-to-photon ratio. Using this value, are the BBN predictions for the abundances of light elements in agreement with the observations?
The present measurement of helium-4 indicates good agreement, and yet better agreement for helium-3. But for lithium-7, there is a significant discrepancy between BBN and WMAP/Planck, and the abundance derived from Population II stars. The discrepancy is a factor of 2.4―4.3 below the theoretically predicted value and is considered a problem for the original models,[14] that have resulted in revised calculations of the standard BBN based on new nuclear data, and to various reevaluation proposals for primordial proton-proton nuclear reactions, especially the abundances of 7Be(n,p)7Li versus 7Be(d,p)8Be.[15]
Non-standard scenarios
In addition to the standard BBN scenario there are numerous non-standard BBN scenarios. These should not be confused with non-standard cosmology: a non-standard BBN scenario assumes that the Big Bang occurred, but inserts additional physics in order to see how this affects elemental abundances. These pieces of additional physics include relaxing or removing the assumption of homogeneity, or inserting new particles such as massive neutrinos.[16]
There have been, and continue to be, various reasons for researching non-standard BBN. The first, which is largely of historical interest, is to resolve inconsistencies between BBN predictions and observations. This has proved to be of limited usefulness in that the inconsistencies were resolved by better observations, and in most cases trying to change BBN resulted in abundances that were more inconsistent with observations rather than less. The second reason for researching non-standard BBN, and largely the focus of non-standard BBN in the early 21st century, is to use BBN to place limits on unknown or speculative physics. For example, standard BBN assumes that no exotic hypothetical particles were involved in BBN. One can insert a hypothetical particle (such as a massive neutrino) and see what has to happen before BBN predicts abundances that are very different from observations. This has been done to put limits on the mass of a stable tau neutrino.
See also
- Big Bang
- Chronology of the universe
- Nucleosynthesis
- Stellar nucleosynthesis
- Ultimate fate of the universe
References
- ↑ Dolgov, A. D. "Big Bang Nucleosynthesis." Nucl.Phys.Proc.Suppl. (2002): 137-43. ArXiv. 17 Jan. 2002. Web. 14 Jan. 2013.
- ↑ Grupen, Claus. "Big Bang Nucleosynthesis." Astroparticle Physics. Berlin: Springer, 2005. 213-28. Print.
- ↑ J. Beringer et al. (Particle Data Group), "Big-Bang cosmology" Phys. Rev. D86, 010001 (2012): (21.43)
- ↑ "Hubble Observations Bring Some Surprises". The New York Times. 1992-01-14. Retrieved 2010-04-26.
- ↑ Gary Steigman (December 2007). "Primordial Nucleosynthesis in the Precision Cosmology Era". Annual Review of Nuclear and Particle Science. 57: 463–491. arXiv:0712.1100
 . Bibcode:2007ARNPS..57..463S. doi:10.1146/annurev.nucl.56.080805.140437.
. Bibcode:2007ARNPS..57..463S. doi:10.1146/annurev.nucl.56.080805.140437. - ↑ Bertulani, Carlos A. (2013). Nuclei in the Cosmos. World Scientific. ISBN 978-981-4417-66-2.
- ↑ Weiss, Achim. "Equilibrium and change: The physics behind Big Bang Nucleosynthesis". Einstein Online. Archived from the original on 8 February 2007. Retrieved 2007-02-24.
- 1 2 http://iopscience.iop.org/article/10.1088/1742-6596/665/1/012001/pdf
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 14–15. ISBN 0-08-037941-9.
- ↑ https://arxiv.org/pdf/1403.4156v1.pdf
- ↑ Bludman, S. A. (December 1998). "Baryonic Mass Fraction in Rich Clusters and the Total Mass Density in the Cosmos". Astrophysical Journal. 508 (2): 535–538. arXiv:astro-ph/9706047
 . Bibcode:1998ApJ...508..535B. doi:10.1086/306412.
. Bibcode:1998ApJ...508..535B. doi:10.1086/306412. - ↑ David Toback (2009). "Chapter 12: Cosmic Background Radiation"
- ↑ David Toback (2009). "Unit 4: The Evolution Of The Universe"
- ↑ R. H. Cyburt, B. D. Fields & K. A. Olive (2008). "A Bitter Pill: The Primordial Lithium Problem Worsens". arXiv:0808.2818
 .
. - ↑ Weiss, Achim. "Elements of the past: Big Bang Nucleosynthesis and observation". Einstein Online. Archived from the original on 8 February 2007. Retrieved 2007-02-24.
For a recent calculation of BBN predictions, see A. Coc; et al. (2004). "Updated Big Bang Nucleosynthesis confronted to WMAP observations and to the Abundance of Light Elements". Astrophysical Journal. 600 (2): 544–552. arXiv:astro-ph/0309480 . Bibcode:2004ApJ...600..544C. doi:10.1086/380121.
. Bibcode:2004ApJ...600..544C. doi:10.1086/380121.
For the observational values, see the following articles:- Helium-4: K. A. Olive & E. A. Skillman (2004). "A Realistic Determination of the Error on the Primordial Helium Abundance". Astrophysical Journal. 617 (1): 29–49. arXiv:astro-ph/0405588
 . Bibcode:2004ApJ...617...29O. doi:10.1086/425170.
. Bibcode:2004ApJ...617...29O. doi:10.1086/425170. - Helium-3: T. M. Bania, R. T. Rood & D. S. Balser (2002). "The cosmological density of baryons from observations of 3He+ in the Milky Way". Nature. 415 (6867): 54–7. Bibcode:2002Natur.415...54B. doi:10.1038/415054a. PMID 11780112.
- Deuterium: J. M. O'Meara; et al. (2001). "The Deuterium to Hydrogen Abundance Ratio Towards a Fourth QSO: HS0105+1619". Astrophysical Journal. 552 (2): 718–730. arXiv:astro-ph/0011179
 . Bibcode:2001ApJ...552..718O. doi:10.1086/320579.
. Bibcode:2001ApJ...552..718O. doi:10.1086/320579. - Lithium-7: C. Charbonnel & F. Primas (2005). "The Lithium Content of the Galactic Halo Stars". Astronomy & Astrophysics. 442 (3): 961–992. arXiv:astro-ph/0505247
 . Bibcode:2005A&A...442..961C. doi:10.1051/0004-6361:20042491. A. Korn; et al. (2006). "A probable stellar solution to the cosmological lithium discrepancy". Nature. 442 (7103): 657–9. arXiv:astro-ph/0608201
. Bibcode:2005A&A...442..961C. doi:10.1051/0004-6361:20042491. A. Korn; et al. (2006). "A probable stellar solution to the cosmological lithium discrepancy". Nature. 442 (7103): 657–9. arXiv:astro-ph/0608201 . Bibcode:2006Natur.442..657K. doi:10.1038/nature05011. PMID 16900193.
. Bibcode:2006Natur.442..657K. doi:10.1038/nature05011. PMID 16900193.
- Helium-4: K. A. Olive & E. A. Skillman (2004). "A Realistic Determination of the Error on the Primordial Helium Abundance". Astrophysical Journal. 617 (1): 29–49. arXiv:astro-ph/0405588
- ↑ Soler, F. J. P., Froggatt, C. D., & Muheim, F., eds., Neutrinos in Particle Physics, Astrophysics and Cosmology (Baton Rouge: CRC Press, 2009), p. 362.
External links
For a general audience
- Weiss, Achim. "Big Bang Nucleosynthesis: Cooking up the first light elements". Einstein Online. Archived from the original on 8 February 2007. Retrieved 2007-02-24.
- White, Martin: Overview of BBN
- Wright, Ned: BBN (cosmology tutorial)
- Big Bang nucleosynthesis on arxiv.org
- Burles, Scott; Nollett, Kenneth M.; Turner, Michael S. (1999-03-19). "Big-Bang Nucleosynthesis: Linking Inner Space and Outer Space". arXiv:astro-ph/9903300
 .
.
Technical articles
- Burles, Scott; Kenneth M. Nollett; Michael S. Turner (2001). "What Is The BBN Prediction for the Baryon Density and How Reliable Is It?". Phys. Rev. D. 63 (6): 063512. arXiv:astro-ph/0008495
 . Bibcode:2001PhRvD..63f3512B. doi:10.1103/PhysRevD.63.063512. Report-no: FERMILAB-Pub-00-239-A
. Bibcode:2001PhRvD..63f3512B. doi:10.1103/PhysRevD.63.063512. Report-no: FERMILAB-Pub-00-239-A - Jedamzik, Karsten, "Non-Standard Big Bang Nucleosynthesis Scenarios". Max-Planck-Institut für Astrophysik, Garching.
- Steigman, Gary, Primordial Nucleosynthesis: Successes And Challenges arXiv:astro-ph/0511534; Forensic Cosmology: Probing Baryons and Neutrinos With BBN and the CBR arXiv:hep-ph/0309347; and Big Bang Nucleosynthesis: Probing the First 20 Minutes arXiv:astro-ph/0307244
- R. A. Alpher, H. A. Bethe, G. Gamow, The Origin of Chemical Elements, Physical Review 73 (1948), 803. The so-called αβγ paper, in which Alpher and Gamow suggested that the light elements were created by hydrogen ions capturing neutrons in the hot, dense early universe. Bethe's name was added for symmetry
- Gamow, G. (1948). "The Origin of Elements and the Separation of Galaxies". Physical Review. 74: 505–506. Bibcode:1948PhRv...74..505G. doi:10.1103/physrev.74.505.2. These two 1948 papers of Gamow laid the foundation for our present understanding of big-bang nucleosynthesis
- Gamow, G. (1948). "The Evolution of the Universe". Nature. 162: 680–2. Bibcode:1948Natur.162..680G. doi:10.1038/162680a0. PMID 18893719.
- Alpher, R. A. (1948). "A Neutron-Capture Theory of the Formation and Relative Abundance of the Elements". Physical Review. 74: 1737–1742. Bibcode:1948PhRv...74.1737A. doi:10.1103/PhysRev.74.1737.
- R. A. Alpher and R. Herman, "On the Relative Abundance of the Elements," Physical Review 74 (1948), 1577. This paper contains the first estimate of the present temperature of the universe
- Alpher, R. A.; Herman, R.; Gamow, G. (1948). "Evolution of the Universe". Nature. 162: 774–775. Bibcode:1948Natur.162..774A. doi:10.1038/162774b0.
- Java Big Bang element abundance calculator