Caesium nitrate
| | |
| Identifiers | |
|---|---|
| 7789-18-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 56425 |
| ECHA InfoCard | 100.029.224 |
| EC Number | 232-146-8 |
| UN number | 1451 |
| |
| |
| Properties | |
| CsNO3 | |
| Molar mass | 194.91 g/mol |
| Appearance | white solid |
| Density | 3.685 g/cm3 |
| Melting point | 414 °C (777 °F; 687 K) |
| Boiling point | decomposes, see text |
| 9.16 g/100 ml (0 °C) 196.8 g/100 ml (100 °C) | |
| Solubility in acetone | soluble |
| Solubility in ethanol | slightly soluble |
| Hazards | |
| GHS pictograms | 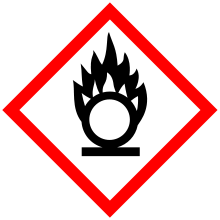 |
| GHS signal word | WARNING |
| H272 | |
| P210, P220, P221, P280, P370+378, P501 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
2390 mg/kg (oral, rat)[2] |
| Related compounds | |
| Other anions |
Caesium nitrite |
| Other cations |
Lithium nitrate Sodium nitrate Potassium nitrate Rubidium nitrate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Caesium nitrate is a chemical compound with the chemical formula CsNO3. An alkali metal nitrate, it is used in pyrotechnic compositions, as a colorant and an oxidizer, e.g. in decoys and illumination flares. The caesium emissions are chiefly due to two powerful spectral lines at 852.113 nm and 894.347 nm.
Caesium nitrate prisms are used in infrared spectroscopy, in x-ray phosphors, and in scintillation counters.[3] It is also used in making optical glasses and lenses.
As with other alkali metal nitrates, caesium nitrate decomposes on gentle heating to give caesium nitrite:
- 2CsNO3 → 2CsNO2 + O2
Caesium also forms two unusual acid nitrates, which can be described as CsNO3·HNO3 and CsNO3·2HNO3 (melting points 100 °C and 36–38 °C respectively).[1]
References
- 1 2 Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-92. ISBN 0-8493-0462-8..
- ↑ http://chem.sis.nlm.nih.gov/chemidplus/rn/7789-18-6
- ↑ Budavari, Susan, ed. (2001), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (13th ed.), Merck, p. 345, ISBN 0911910131.
External links
| Salts and covalent derivatives of the Nitrate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HNO3 | He | ||||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)4− | C | N | O | FNO3 | Ne | ||||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3 | Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr | ||
| RbNO3 | Sr(NO3)2 | Y | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb | Te | I | Xe(NO3)2 | ||
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3 | Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd | Pm | Sm | Eu | Gd(NO3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from Wikipedia - version of the 9/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.