Chromium nitrate
 | |
| Names | |
|---|---|
| IUPAC name
Chromium(III) nitrate | |
| Other names
Nitric acid, chromium(3+) salt | |
| Identifiers | |
| 13548-38-4 7789-02-8 (nonahydrate) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 15285818 |
| ECHA InfoCard | 100.033.550 |
| PubChem | 24598 |
| RTECS number | GB6300000 |
| UNII | C6H0RE016B |
| UN number | 2720 |
| |
| |
| Properties | |
| Cr(NO3)3 [Cr(H2O)6](NO3)3•3H2O (nonahydrate) | |
| Molar mass | 238.011 g/mol (anhydrous) 400.21 g/mol (nonahydrate) |
| Appearance | Blue-violet crystals (anhydrous) Purple crystals (nonahydrate) |
| Density | 1.85 g/cm3 (nonahydrate) |
| Melting point | 60.06 °C (140.11 °F; 333.21 K) nonahydrate |
| Boiling point | > 100 °C (212 °F; 373 K) (decomposes) |
| 81 g/100 mL (20 °C) | |
| Hazards | |
| Safety data sheet | Oxford MSDS |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
3250 mg/kg (rat, oral, nonahydrate) 110 mg/kg (mouse, oral)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Chromium(III) nitrate describes several inorganic compounds consisting of chromium, nitrate and varying amounts of water. Most common is the dark violet hydrated solid, but an anhydrous green form is also known. Chromium(III) nitrate compounds are of a limited importance commercially, finding some applications in the dyeing industry.[2] It is common in academic laboratories for the synthesis of chromium coordination complexes.
Structure
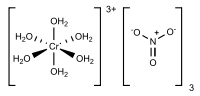
The relatively complicated formula - [Cr(H2O)6](NO3)3•3H2O - highlights the complicated structure of this material. The chromium centers are bound to six water ligands, and the remaining volume of the solid is occupied by three nitrate anions and three molecules of water of crystallization. Such complicated formulas typify hydrated metal salts.
Properties and preparation
The anhydrous salt forms green crystals and very soluble in water (in contrast to anhydrous chromium(III) chloride which dissolves very slowly except under special conditions). At 100 °C it decomposes. The red-violet hydrate is highly soluble in water. Chromium nitrate is used in the production of alkali metal-free catalysts and in pickling.
Chromium nitrate can be prepared by dissolving chromium oxide in nitric acid.[2]
References
- ↑ "Chromium(III) compounds [as Cr(III)]". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
| Salts and covalent derivatives of the Nitrate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HNO3 | He | ||||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)4− | C | N | O | FNO3 | Ne | ||||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3 | Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr | ||
| RbNO3 | Sr(NO3)2 | Y | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb | Te | I | Xe(NO3)2 | ||
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3 | Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd | Pm | Sm | Eu | Gd(NO3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
