Calcium fluoride
| |||
| | |||
| Identifiers | |||
|---|---|---|---|
| 7789-75-5 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:35437 | ||
| ChemSpider | 23019 | ||
| ECHA InfoCard | 100.029.262 | ||
| EC Number | 232-188-7 | ||
| PubChem | 24617 | ||
| RTECS number | EW1760000 | ||
| UNII | O3B55K4YKI | ||
| |||
| |||
| Properties | |||
| CaF2 | |||
| Molar mass | 78.07 g·mol−1 | ||
| Appearance | White crystalline solid (single crystals are transparent) | ||
| Density | 3.18 g/cm3 | ||
| Melting point | 1,418 °C (2,584 °F; 1,691 K) | ||
| Boiling point | 2,533 °C (4,591 °F; 2,806 K) | ||
| 0.0015 g/100 mL (18 °C) 0.0016 g/100 mL (20 °C) | |||
| Solubility product (Ksp) |
3.9 × 10−11 [1] | ||
| Solubility | insoluble in acetone slightly soluble in acid | ||
| Refractive index (nD) |
1.4338 | ||
| Structure | |||
| cubic crystal system, cF12[2] | |||
| Fm3m, #225 | |||
| Ca, 8, cubic F, 4, tetrahedral | |||
| Hazards | |||
| Main hazards | Reacts with conc. sulfuric acid to produce hydrofluoric acid | ||
| Safety data sheet | ICSC 1323 | ||
| R-phrases | R20, R22, R36, R37, R38 | ||
| S-phrases | S26, S36 | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
| LDLo (lowest published) |
>5000 mg/kg (oral, guinea pig) 4250 mg/kg (oral, rat)[3] | ||
| Related compounds | |||
| Other anions |
Calcium chloride Calcium bromide Calcium iodide | ||
| Other cations |
Beryllium fluoride Magnesium fluoride Strontium fluoride Barium fluoride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Calcium fluoride is the inorganic compound with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.
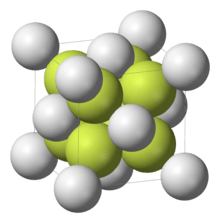
Chemical structure
The compound crystallizes in a cubic motif called a fluorite structure. Ca2+ centres are eight-coordinate, being centered in a "box" for eight F− centres. Each F− centre is coordinated to four Ca2+ centre.[4] Although perfectly packed crystalline samples are colorless, the mineral is often deeply colored due to the presence of F-centers.
Preparation
The mineral fluorite is abundant, widespread, and mainly of interest as a precursor to HF. Thus, little motivation exists for the industrial production of CaF2. High purity CaF2 is produced by treating calcium carbonate with hydrofluoric acid:[5]
- CaCO3 + 2 HF → CaF2 + CO2 + H2O
Applications
Naturally occurring CaF2 is the principal source of hydrogen fluoride, a commodity chemical used to produce a wide range of materials. Calcium fluoride in the fluorite state is of significant commercial importance as a fluoride source.[6] Hydrogen fluoride is liberated from the mineral by the action of concentrated sulfuric acid:[7]
- CaF2 + H2SO4 → CaSO4(solid) + 2 HF
Niche uses
Calcium fluoride is used to manufacture optical components such as windows and lenses, used in thermal imaging systems, spectroscopy, and excimer lasers. It is transparent over a broad range from ultraviolet (UV) to infrared (IR) frequencies. Its low refractive index reduces the need for anti-reflection coatings. Its insolubility in water is convenient as well.
Safety
CaF2 is classified "not dangerous", although reacting it with sulfuric acid produces toxic hydrofluoric acid. With regards to inhalation, the NIOSH-recommended concentration of fluorine-containing dusts is 2.5 mg/m3 in air.[5]
References
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑ X-ray Diffraction Investigations of CaF2 at High Pressure, L. Gerward, J. S. Olsen, S. Steenstrup, M. Malinowski, S. Åsbrink and A. Waskowska, Journal of Applied Crystallography (1992), 25, 578-581 doi:10.1107/S0021889892004096
- ↑ "Fluorides (as F)". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ G. L. Miessler and D. A. Tarr “Inorganic Chemistry” 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- 1 2 Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, René; Cuer, Jean Pierre (2000). "Fluorine Compounds, Inorganic". doi:10.1002/14356007.a11_307.
- ↑ Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, Renée; Cuer, Jean Pierre (2005), "Fluorine Compounds, Inorganic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, p. 307, doi:10.1002/14356007.a11_307.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
See also
Related materials
External links
- NIST webbook thermochemistry data
- Charles Townes on the history of lasers
- National Pollutant Inventory - Fluoride and compounds fact sheet
- Crystran Material Data
- MSDS (University of Oxford)