Sodium hexafluoroaluminate
 | |
 | |
| Names | |
|---|---|
| Other names
Sodium fluoroaluminate Cryolite Kryolite Aluminate(3-), hexafluoro-, trisodium, (OC-6-11)- | |
| Identifiers | |
| 13775-53-6 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:39289 |
| ChemSpider | 11431435 |
| ECHA InfoCard | 100.035.575 |
| PubChem | 159692 |
| |
| |
| Properties | |
| Na3AlF6 | |
| Molar mass | 209.94 g/mol |
| Appearance | white powder |
| Density | 2.9 g/cm3, solid |
| Melting point | 950 °C (1,740 °F; 1,220 K) |
| Boiling point | decomposes |
| 0.04% (20°C)[1] | |
| Vapor pressure | essentially 0 |
| Hazards | |
| EU classification (DSD) |
not listed |
| Lethal dose or concentration (LD, LC): | |
| LDLo (lowest published) |
900 mg/kg (rabbit, oral)[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 2.5 mg/m3[1] |
| REL (Recommended) |
TWA 2.5 mg/m3[1] |
| IDLH (Immediate danger) |
250 mg/m3 (as F)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
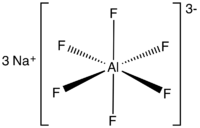
Sodium aluminium hexafluoride is the inorganic compound with the formula Na3AlF6. This white solid occurs naturally as the mineral cryolite and is used extensively in the industrial production of aluminium metal. The compound consists of the sodium (Na+) salt of hexafluoroaluminate (AlF63-).
Production
Most cryolite is manufactured from aluminium oxides, hydrofluoric acid, and sodium hydroxide or the equivalent reagent hexafluorosilicic acid:[3]
- 6 NaOH + Al2O3 + 12 HF → 2 Na3AlF6 + 9 H2O
Use
The main application of synthetic cryolite is as a solvent (or flux) for electrolysis aluminium oxides such as bauxite. The conversion of aluminium oxides into metallic aluminium requires that the metal ions be dissolved so that they can accept the electrons provided in the electrolysis cell. A mixture of cryolite and some aluminium trifluoride is that solvent. Unlike typical solutions, this one requires temperatures approaching 1000 °C to melt. Sodium aluminium hexafluoride is also used as a pesticide. Other uses include a whitener for enamels and an opacifier for glass. [4]
Safety
Cryolite is poorly soluble in water which mitigates problems. Upon ingestion, however, the acids in the stomach increases this solubility. The LD50 = 200 mg/kg, comparable to that for soluble fluoride salts. In 1957, sodium aluminium hexafluoride was registered as a pesticide with the United States EPA. Four Sodium hexafluoroaluminate products registered.[5]
References
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0559". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Fluorides (as F)". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ J. Aigueperse, P. Mollard, D. Devilliers, M. Chemla, R. Faron, R. Romano, J. P. Cuer, "Fluorine Compounds, Inorganic" in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a11_307
- ↑ http://www.mineralszone.com/minerals/cryolite.html
- ↑ http://www.epa.gov/oppsrrd1/REDs/0087.pdf