Cryptosporidium
| Cryptosporidium | |
|---|---|
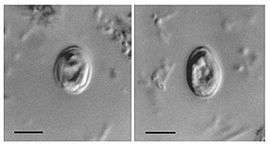 | |
| Cryptosporidium muris oocysts found in human feces. | |
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | Sar |
| (unranked): | Alveolata |
| Phylum: | Apicomplexa |
| Class: | Conoidasida |
| Genus: | Cryptosporidium Tyzzer, 1907 |
| Species | |
|
Cryptosporidium andersoni | |
Cryptosporidium is a genus of apicomplexan parasitic protozoans that can cause a respiratory and gastrointestinal illness (cryptosporidiosis) that primarily involves watery diarrhea (intestinal cryptosporidiosis) with or without a persistent cough (respiratory cryptosporidiosis) in both immunocompetent and immunodeficient humans.[1]
Treatment of gastrointestinal infection in humans involves fluid rehydration, electrolyte replacement, and management of any pain. As of January 2015, nitazoxanide is the only drug approved for the treatment of cryptosporidiosis in immunocompetent hosts.[2] Supplemental zinc may improve symptoms,[2] particularly in recurrent or persistent infections or in others at risk for zinc deficiency. Cryptosporidium oocysts are 4–6 µm in diameter and exhibit partial acid-fast staining. They must be differentiated from other partially acid-fast organisms including Cyclospora cayetanensis.
General characteristics
Cryptosporidium causes cryptosporidiosis, an infection that may present as a diarrhoeal with or without a persistent cough in immunocompetent hosts.[1] Other apicomplexan pathogens include the malaria parasite Plasmodium, and the toxoplasmosis parasite Toxoplasma. Unlike Plasmodium, which transmits via a mosquito vector, Cryptosporidium does not use an insect vector, and is capable of completing its lifecycle within a single host, resulting in cyst stages that are excreted in feces or through coughing fomites and are capable of transmission to a new host.[1][3]
A number of Cryptosporidium species infect mammals. In humans, the main causes of disease are C. parvum and C. hominis (previously C. parvum genotype 1). C. canis, C. felis, C. meleagridis, and C. muris can also cause disease in humans.[3]
Cryptosporidiosis is typically an acute, short-term infection, can be recurrent through reinfection in immunocompetent hosts, and become severe or life-threatening in immunocompromised individuals. In humans, it remains in the lower intestine and may remain for up to five weeks.[3] The parasite is transmitted by environmentally hardy cysts (oocysts) that, once ingested, exist in the small intestine and result in an infection of intestinal epithelial tissue.[3] Transmission by ingestion or inhalation of coughed fomites is a second, less likely route of infection.[1]
The genome of Cryptosporidium parvum, sequenced in 2004, was found to be unusual amongst eukaryotes in that the mitochondria seem not to contain DNA.[4] A closely related species, C. hominis, also has its genome sequence available.[5]
Life cycle

The Cryptosporidium spore phase (oocyst) can survive for lengthy periods outside a host. It can also resist many common disinfectants, notably chlorine-based disinfectants.[6]
Water treatment and detection
Many treatment plants that take raw water from rivers, lakes, and reservoirs for public drinking water production use conventional filtration technologies. Direct filtration, which is typically used to treat water with low particulate levels, includes coagulation and filtration but not sedimentation. Other common filtration processes including slow sand filters, diatomaceous earth filter, and membranes will remove 99% of Cryptosporidium.[7] Membranes and bag- and cartridge-filter products remove Cryptosporidium specifically.
Cryptosporidium is highly resistant to chlorine disinfection;[8] but with high enough concentrations and contact time, Cryptosporidium inactivation will occur with chlorine dioxide and ozone treatment. In general, the required levels of chlorine preclude the use of chlorine disinfection as a reliable method to control Cryptosporidium in drinking water. Ultraviolet light treatment at relatively low doses will inactivate Cryptosporidium. Water Research Foundation-funded research originally discovered UV's efficacy in inactivating Cryptosporidium.[9][10]
One of the largest challenges in identifying outbreaks is the ability to verify the results in a laboratory. The oocytes may be seen by microscopic examination of a stool sample, but they may be confused with other objects or artifacts similar in appearance.[11] Most cryptosporidia are 3–6 μm in size, although some reports have described larger cells.[11] Real-time monitoring technology is now able to detect Cryptosporidium with online systems versus the spot testing and batch testing methods used in the past.
For the end consumer of drinking water believed to be contaminated by Cryptosporidium, the safest option is to boil all water used for drinking.[12][13]
Exposure risks
The following groups have an elevated risk of being exposed to Cryptosporidium:
- People who swim regularly in pools with insufficient sanitation (Certain strains of Cryptosporidium are chlorine-resistant)
- Child-care workers
- Parents of infected children
- People caring for other people with cryptosporidiosis
- Backpackers, hikers, and campers who drink unfiltered, untreated water
- People, including swimmers, who swallow water from contaminated sources
- People handling infected cattle
- People exposed to human faeces
Cases of cryptosporidiosis can occur in a city with clean water; cases of cryptosporidiosis can have different origins. Like many fecal-oral pathogens, it can also be transmitted by contaminated food or poor hygiene. Testing of water, as well as epidemiological study, are necessary to determine the sources of specific infections. Cryptosporidium typically does not cause serious illness in healthy people. It may chronically sicken some children, as well as adults exposed and immunocompromised. A subset of the immunocompromised population is people with AIDS, amongst MSM with AIDS insertive anal sex is an increased risk factor.[14] Analingus, oral–genital sex after anal–genital sex are known transmission routes.[15]
Other transmission routes include exposure to laboratory specimens.[15]
See also
- 1987 Carroll County Cryptosporidiosis outbreak
- 1993 Milwaukee Cryptosporidiosis outbreak
- 1998 Sydney water crisis
- Escherichia coli
- Giardia lamblia
References
- 1 2 3 4 Sponseller JK, Griffiths JK, Tzipori S (2014). "The evolution of respiratory Cryptosporidiosis: evidence for transmission by inhalation". Clin. Microbiol. Rev. 27 (3): 575–86. doi:10.1128/CMR.00115-13. PMC 4135895
 . PMID 24982322.
. PMID 24982322. Recent evidence indicates that respiratory cryptosporidiosis may occur commonly in immunocompetent children with cryptosporidial diarrhea and unexplained cough. Findings from animal models, human case reports, and a few epidemiological studies suggest that Cryptosporidium may be transmitted via respiratory secretions, in addition to the more recognized fecal-oral route. ... Upper respiratory cryptosporidiosis may cause inflammation of the nasal mucosa, sinuses, larynx, and trachea, accompanied by nasal discharge and voice change (54, 61, 62). Cryptosporidiosis of the lower respiratory tract typically results in productive cough, dyspnea, fever, and hypoxemia (63,–66). ... While fecal-oral transmission is indisputably the major route of infection, transmission via coughing and fomites is also possible in situations of close contact (20). ... Because they lacked gastrointestinal symptoms and oocyst excretion, the latter cases establish the possibility of primary respiratory infection with Cryptosporidium, which may have been acquired by inhalation of expectorated droplets or by contact with fomites. ... This finding suggests that respiratory cryptosporidiosis may occur commonly in immunocompetent individuals.
- 1 2 Cabada MM, White AC, Venugopalan P, Sureshbabu J (18 August 2015). Bronze MS, ed. "Cryptosporidiosis Treatment & Management". Medscape. WebMD. Retrieved 8 January 2016.
Infection may improve with nutritional supplementation, particularly with regimens including zinc or glutamine. ... Nitazoxanide significantly shortens the duration of diarrhea and can decrease the risk of mortality in malnourished children.[22] Trials have also demonstrated efficacy in adults.[26, 27] ... Symptomatic therapy includes replacement of fluids, provision of appropriate nutrition, and treatment with antimotility agents. ... Replacement of fluids and electrolytes is the critically important first step in the management of cryptosporidiosis, particularly in patients with large diarrheal losses. Fluids should include sodium, potassium, bicarbonate, and glucose.
- 1 2 3 4 http://www.cdc.gov/parasites/crypto/pathogen.html
- ↑ Abrahamsen, Mitchell S.; Templeton, Thomas J.; Enomoto, Shinichiro; Abrahante, Juan E.; Zhu, Guan; Lancto, Cheryl A.; Deng, Mingqi; Liu, Chang; Widmer, Giovanni; Tzipori, Saul; Buck, Gregory A.; Xu, Ping; Bankier, Alan T.; Dear, Paul H.; Konfortov, Bernard A.; Spriggs, Helen F.; Iyer, Lakshminarayan; Anantharaman, Vivek; Aravind, L.; Kapur, Vivek (2004). "Complete Genome Sequence of the Apicomplexan, Cryptosporidium parvum". Science. 304 (5669): 441–5. doi:10.1126/science.1094786. PMID 15044751.
- ↑ Xu, Ping; Widmer, Giovanni; Wang, Yingping; Ozaki, Luiz S.; Alves, Joao M.; Serrano, Myrna G.; Puiu, Daniela; Manque, Patricio; Akiyoshi, Donna; Mackey, Aaron J.; Pearson, William R.; Dear, Paul H.; Bankier, Alan T.; Peterson, Darrell L.; Abrahamsen, Mitchell S.; Kapur, Vivek; Tzipori, Saul; Buck, Gregory A. (2004). "The genome of Cryptosporidium hominis". Nature. 431 (7012): 1107–12. doi:10.1038/nature02977. PMID 15510150.
- ↑ "Chlorine Disinfection of Recreational Water for Cryptosporidium parvum". CDC. Retrieved 2007-05-06.
- ↑ "The Interim Enhanced Surface Water Treatment Rule – What Does it Mean to You?" (PDF). USEPA. Retrieved 2007-05-06.
- ↑ Korich, DG; Mead, JR; Madore, MS; Sinclair, NA; Sterling, CR (1990). "Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability". Applied and Environmental Microbiology. 56 (5): 1423–8. PMC 184422
 . PMID 2339894.
. PMID 2339894. - ↑ Rochelle, Paul A.; Fallar, Daffodil; Marshall, Marilyn M.; Montelone, Beth A.; Upton, Steve J.; Woods, Keith (2004). "Irreversible UV Inactivation of Cryptosporidium spp. Despite the Presence of UV Repair Genes". The Journal of Eukaryotic Microbiology. 51 (5): 553–62. doi:10.1111/j.1550-7408.2004.tb00291.x. PMID 15537090.
- ↑ "Ultraviolet Disinfection and Treatment". WaterResearchFoundation (formerly AwwaRF). Retrieved 2007-05-06.
- 1 2 Casemore, D P; Armstrong, M; Sands, R L (1985). "Laboratory diagnosis of cryptosporidiosis". Journal of Clinical Pathology. 38 (12): 1337–41. doi:10.1136/jcp.38.12.1337. PMC 499488
 . PMID 2416782.
. PMID 2416782. - ↑ http://news.bbc.co.uk/1/hi/wales/7589839.stm[]
- ↑ http://news.bbc.co.uk/1/hi/wales/4484946.stm[]
- ↑ http://sti.bmj.com/content/79/5/412.full
- 1 2 Kenneth A. Borchardt; Michael A. Noble (25 June 1997). Sexually Transmitted Diseases: Epidemiology, Pathology, Diagnosis, and Treatment. CRC Press. p. 192. ISBN 978-0-8493-9476-8.
Further reading
- White, A. Clinton, Jr. "Cryptosporidiosis". In Mandell, G et al., eds., Principles and Practice of Infectious Diseases, 6th edition; Elsevier, 2005, pp 3215–28.
- Upton, Steve J. (2003-09-12). "Basic Biology of Cryptosporidium" (Website). Kansas State University: Parasitology Laboratory.
- "The Taxonomicon & Systema Naturae" (Website database). Taxon: Genus Cryptosporidium. S.J. Brands (Compiler). Universal Taxonomic Services, Amsterdam, The Netherlands. 2000.
- United States Environmental Protection Agency (March 2001). "Cryptosporidium: Drinking Water Advisory" (PDF). Retrieved 4 March 2013 EPA reference: EPA-822-R-01-009
- World Health Organisation (2009). "Risk Assessment of Cryptosporidium in Drinking Water" (PDF). Retrieved 4 March 2013 WHO reference: WHO/HSE/WSH/09.04