Dehydrogenase
A dehydrogenase (also called DH or DHase in the literature) is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by a reduction reaction that removes one or more hydrogens from a substrate to an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN.
Enzyme class
Dehydrogenases are a subclass of the class of enzymes labeled “oxidoreductases.” Oxidoreductases, in general, catalyze oxidation and reduction reactions. Any enzyme that transfers an electron from one molecule to another is considered an oxidoreductase. These enzymes fall into six categories: oxygenases, reductases, peroxidases, oxidases, hydroxylases, and dehydrogenases. Most oxidoreductase enzymes are dehydrogenases, although reductases are also common. Accepted nomenclature for dehydrogenases is "donor dehydrogenase," where the donor is the molecule giving up an electron.[1]
Oxidation-reduction reactions are essential to growth and survival of organisms, as the oxidation of carbons produces energy. Energy-producing reactions can drive forward the synthesis of important energy molecules, such as ATP in glycolysis. For this reason, dehydrogenases have pivotal roles in metabolism.[2]
Reactions catalyzed
Dehydrogenases oxidize a substrate by transferring a hydrogen to an electron acceptor, common electron acceptors being NAD+ or FAD. This would be considered an oxidation of the substrate, in which the substrate is losing electrons.[3] When considered, the name "dehydrogenase" is a logical moniker for this enzyme, as it facilitates the removal (de-) of a hydrogen (-hydrogen-), by an enzyme (-ase). In contrast, reductases are a subclass of oxidoreductases that catalyze reduction reactions, or a reaction in which a compound is gaining electrons.[3] Dehydrogenase reactions come most commonly in two forms: the transfer of a hydride and release of a proton, and the transfer of two hydrogens.
Transferring a hydride and releasing a proton
Typically, a dehydrogenase catalyzed reaction will look like this: AH + B ↔ A− + BH when a hydride is transferred.
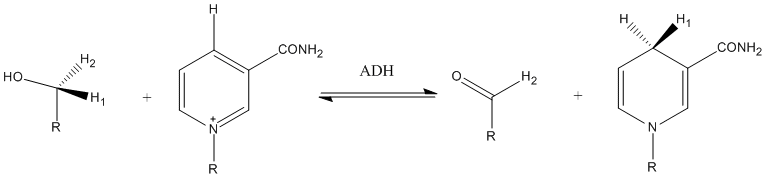
A represents the substrate that will be oxidized, while B is the hydride acceptor.[4] Note how when the hydride is transferred from A to B, the A has taken on a positive charge; this is because the enzyme has taken two electrons from the substrate in order to reduce the acceptor to BH.[2]
Acquiring a positive charge is not always the result of a dehydrogenase catalyzed reaction, often the free electrons on the substrate are moved into a double bond. This happens frequently when an alcohol (OH) is the substrate; when the hydrogen is removed the free electrons on the oxygen will be used to create a double bond, as seen in the oxidation of ethanol to acetaldehyde carried out by alcohol dehydrogenase in the image on the right.[5]
.png)
Transferring two hydrogens
In the above case, the dehydrogenase has transferred a hydride while releasing a proton, H+, but dehydrogenases can also transfer two hydrogens, using FAD as an electron acceptor. This would be depicted as AH2 + B ↔ A− + BH2.
The negative charge on the substrate is often not seen, as when two hydrogens are transferred a double bond is normally formed in between the two atoms that the hydrogens were taken from, as in the case of succinate dehydrogenase. The two hydrogens have been transferred to the carrier or the other product, along with the two electrons.
In this case (and more commonly) the reaction would show as AH2 + B ↔ A + BH2. Where a double bond is formed in the compound A, shifting its excess electrons into the bond. A common acceptor in this case is FAD, discussed later.
Identifying a dehydrogenase reaction
Given the similarity between the subclasses of oxidoreductases, it is crucial to properly understand what divides dehydrogenase from any differing oxidoreductase. Differences between dehydrogenases and reductases are clear: reductases will add electrons to a substrate while dehydrogenases remove them. The distinction between the subclasses of oxidoreductases that catalyze oxidation reactions lies in their electron acceptors.[1]
Dehydrogenase and oxidase are easily distinguishable if one considers the electron acceptor. An oxidase will remove electrons from a substrate as well, but only uses oxygen as its electron acceptor, a general outline of an oxidase reaction looks like this: AH + O2 ↔ A + H2O2.
More commonly, an oxidase reaction will look like this: 2A + 4H+ + O2 ↔ 2A+ + 2H2O. In this case, the enzyme is taking electrons from the substrate, and using free hydrogens to reduce the oxygen, leaving the substrate with a positive charge. The product is water, instead of hydrogen peroxide as seen above.[2] An example of an oxidase that functions like this is complex IV in the Electron Transport Chain (ETC).[6]
Note that oxidases typically transfer dihydrogen (H2), and the acceptor is a dioxygen. Similarly, a peroxidase (another subclass of oxidoreductases) will use a peroxide (H2O2) as the electron acceptor, rather than an oxygen.[5]
Electron acceptors
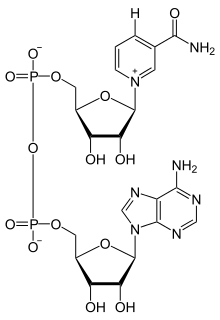
Dehydrogenase enzymes transfer electrons from the substrate to an electron carrier; what carrier is used depends on the reaction taking place. Common electron acceptors used by this subclass are NAD+, FAD, and NADP+. Electron carriers are reduced in this process and considered oxidizers of the substrate. Electron carriers are coenzymes that are often referred to as "redox cofactors."[1]
NAD+
NAD+, or nicotinamide adenine dinucleotide, is a dinucleotide, containing two nucleotides. One of the nucleotides it contains is an adenine group, while the other is nicotinamide. In order to reduce this molecule, a hydrogen and two electrons must be added to the 6-carbon ring of nicotinamide; one electron is added to the carbon opposite the positively charged nitrogen, causing a rearrangement of bonds within the ring to give nitrogen more electrons; it will lose its positive charge as a result. The extra electron is "stolen" from an additional hydrogen, leaving the hydrogen ion in solution.[1][7]
Reduction of NAD+: NAD+ + 2H+ + 2e− ↔ NADH + H+
NAD+ is mostly used in catabolic pathways, such as glycolysis, that break down energy molecules to produce ATP. The ratio of NAD+ to NADH is kept very high in the cell, keeping it readily available to act as an oxidizing agent.[7][8]
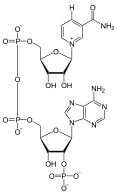
NADP+
NADP+ differs from NAD+ in only the addition of a phosphate group to the adenosine 5-membered carbon ring. The addition of the phosphate does not alter the electron transport abilities of the carrier, but instead facilitates cell distinction between this coenzyme and NAD+. The phosphate group creates enough contrast between the two groups that they bind to the active site of different enzymes, generally catalyzing different types of reactions.[8][9]
It is important that these two electron carriers are easily distinguished, because they participate in very different reactions. NADP+ mainly functions with enzymes that catalyze anabolic, or biosynthetic, pathways.[9] Specifically, NADPH will act as a reducing agent in these reactions, resulting in NADP+. These are pathways that convert substrates to more complicated products, using ATP. The reasoning behind having two separate electron carriers for anabolic and catabolic pathways relates to regulation of metabolism.[7]
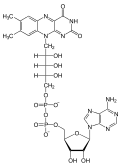
The ratio of NADP+ to NADPH in the cell is kept rather low, so that NADPH is readily available as a reducing agent; it is more commonly used as a reducing agent than NADP+ is used as an oxidizing agent.[8]
FAD
FAD, or flavin adenine dinucleotide, is a prosthetic group (a non-polypeptide unit bound to a protein that is required for function) that consists of an adenine nucleotide and a flavin mononucleotide.[10] FAD is a unique electron acceptor. Its fully oxidized form is FADH2 (known as the hydroquinone form), but FAD can also be partially oxidized as FADH by either reducing FAD or oxidizing FADH2.[11] Dehydrogenases typically fully reduce FAD to FADH2. The production of FADH is rare.
The double-bonded nitrogen atoms in FAD make it a good acceptor in taking two hydrogen atoms from a substrate. Because it takes two atoms rather than one, FAD is often involved when a double bond is formed in the newly oxidized substrate.[12] FAD is unique because it is reduced by two electrons and two protons, as opposed to both NAD+ and NADP, which only take one proton.
Examples
Biological implications

Aldehydes are the natural by-product of many physiological processes, as well as being the consequence of many industrial processes, put out into the environment in the form of smog and motor vehicle exhaust. Build-up of aldehydes in the brain and pericardium can be detrimental to a person's health, as they can form adducts with important molecules and cause their inactivation.[13]
Considering how prevalent aldehydes are, there must be an enzyme to facilitate their oxidation to a less volatile compound. Aldehyde dehydrogenases (ALDH) are NAD+ dependent enzymes that function to remove toxic aldehydes from the body, functioning mostly in the mitochondria of cells. These enzymes are largely responsible for the detoxification of acetylaldehyde, which is an intermediate in the metabolism of ethanol. It has been shown that a mutation in the ALDH2 gene (one of 19 aldehyde dehydrogenase genes) is what leads to the common occurrence in East Asian population of a flushed face after consuming alcohol, due to the build-up of acetaldehyde.[14] This build-up of acetaldehyde also causes headaches and vomiting (sometimes referred to as a "hangover") if not broken down quickly enough, another reason why those with acetaldehyde DH deficiencies have bad reactions to alcohol.[15] Importantly, a lack of this enzyme has been linked to an increase in the risk of myocardial infarction, while activation has shown the enzyme's ability to reduce damage caused by ischaemia.[13]
Deactivation of aldehyde dehydrogenases has been shown to be instrumental in the mechanisms of many cancers. ALDHs function in cell differentiation, proliferation, oxidation, and drug resistance.[16] These enzymes are only one example of the many different types of dehydrogenases in the human body; their wide array of functions, and the impact that their deactivation or mutations has upon crucial cell processes underscores the importance of all dehydrogenases in maintaining body homeostasis.
More examples
- acetaldehyde dehydrogenase
- alcohol dehydrogenase
- glutamate dehydrogenase (an enzyme that can convert glutamate to α-Ketoglutarate and vice versa).
- lactate dehydrogenase (mostly used to convert NADH back to NAD+ in anaerobic glycolysis, but also used in the back reaction to produce NADH)
- pyruvate dehydrogenase (a common enzyme that feeds the TCA Cycle in converting Pyruvate to Acetyl CoA, using NAD+)
- glucose-6-phosphate dehydrogenase (involved in the pentose phosphate pathway, producing NADPH)
- glyceraldehyde-3-phosphate dehydrogenase (involved in glycolysis, uses NAD+)
- sorbitol dehydrogenase
TCA cycle examples:
- isocitrate dehydrogenase (uses NAD+, also has an isozyme that uses NADP)
- alpha-ketoglutarate dehydrogenase (uses NAD+)
- succinate dehydrogenase (uses FAD)
- malate dehydrogenase (uses NAD+)
References
- 1 2 3 4 Voet, Donald (2006). Fundamentals of Biochemistry: Life at the Molecular Level. New York: Wiley.
- 1 2 3 "Oxidative Reactions: Dehydrogenase and Oxidases - BioWiki". biowiki.ucdavis.edu. Retrieved 2016-02-21.
- 1 2 Clark, Jim (2002). "Definitions of Oxidation and Reduction (Redox)". Chemguide. Retrieved February 14, 2016.
- ↑ "What are Oxidoreductases?". www.chem.uwec.edu. Retrieved 2016-02-02.
- 1 2 "Six Major Classes of Enzymes and Examples of Their Subclasses" (PDF).
- ↑ Yoshikawa, Shinya; Shimada, Atsuhiro (2015-01-20). "Reaction Mechanism of Cytochrome c Oxidase". Chemical Reviews. 115 (4): 1936–1989. doi:10.1021/cr500266a. PMID 25603498.
- 1 2 3 Alberts, B; Johnson, A; et al. (2002). Molecular Biology of the Cell. New York: Garland Science. ISBN 0-8153-3218-1.
- 1 2 3 Ying, Weihai (2008-02-01). "NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences". Antioxidants & Redox Signaling. 10 (2): 179–206. doi:10.1089/ars.2007.1672. ISSN 1523-0864. PMID 18020963.
- 1 2 "The physiological role of NADPH". watcut.uwaterloo.ca. Retrieved 2016-03-06.
- ↑ Dym, Orly; Eisenberg, David (2001-09-01). "Sequence-structure analysis of FAD-containing proteins". Protein Science. 10 (9): 1712–1728. doi:10.1110/ps.12801. ISSN 1469-896X. PMC 2253189
 . PMID 11514662.
. PMID 11514662. - ↑ Rivlin, Richard S. (1970-08-27). "Riboflavin Metabolism". New England Journal of Medicine. 283 (9): 463–472. doi:10.1056/NEJM197008272830906. ISSN 0028-4793. PMID 4915004.
- ↑ "blobs.org - Metabolism". www.blobs.org. Retrieved 2016-03-01.
- 1 2 Chen, Che-Hong; Sun, Lihan; Mochly-Rosen, Daria (2010-10-01). "Mitochondrial aldehyde dehydrogenase and cardiac diseases". Cardiovascular Research. 88 (1): 51–57. doi:10.1093/cvr/cvq192. ISSN 0008-6363. PMC 2936126
 . PMID 20558439.
. PMID 20558439. - ↑ Goedde, HW; Agarwal, DP (1983). "Population genetic studies on aldehyde dehydrogenase isozyme deficiency and alcohol sensitivity". Am J Hum Genet. 35 (4): 769–72. PMC 1685745
 . PMID 6881146.
. PMID 6881146. - ↑ "How Hangovers Work". HowStuffWorks. Retrieved 2016-03-06.
- ↑ van den Hoogen, Christel; van der Horst, Geertje; Cheung, Henry; Buijs, Jeroen T.; Lippitt, Jenny M.; Guzmán-Ramírez, Natalia; Hamdy, Freddie C.; Eaton, Colby L.; Thalmann, George N. (2010-06-15). "High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer". Cancer Research. 70 (12): 5163–5173. doi:10.1158/0008-5472.CAN-09-3806. ISSN 1538-7445. PMID 20516116.