Directed evolution
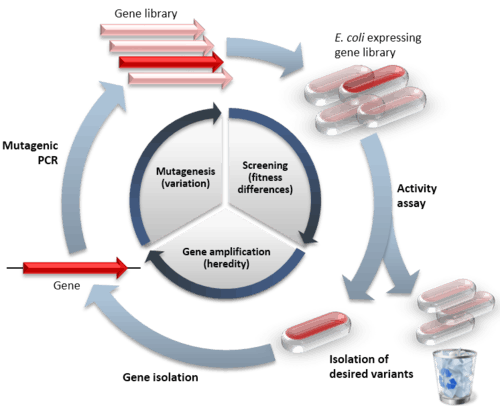
Directed evolution (DE) is a method used in protein engineering that mimics the process of natural selection to evolve proteins or nucleic acids toward a user-defined goal.[1] It consists of subjecting a gene to iterative rounds of mutagenesis (creating a library of variants), selection (expressing the variants and isolating members with the desired function), and amplification (generating a template for the next round). It can be performed in vivo (in living cells), or in vitro (free in solution or microdroplet). Directed evolution is used both for protein engineering as an alternative to rationally designing modified proteins, as well as studies of fundamental evolutionary principles in a controlled, laboratory environment.
Principles

Directed evolution is a mimic of the natural evolution cycle in a laboratory setting. Evolution requires three things to occur: variation between replicators, that the variation causes fitness differences upon which selection acts, and that this variation is heritable. In DE, a single gene is evolved by iterative rounds of mutagenesis, selection or screening, and amplification.[2] Rounds of these steps are typically repeated, using the best variant from one round as the template for the next to achieve stepwise improvements.
The likelihood of success in a directed evolution experiment is directly related to the total library size, as evaluating more mutants increases the chances of finding one with the desired properties.[3]
Generating variation

The first step in performing a cycle of directed evolution is the generation of a library of variant genes. The sequence space for random sequence is vast (10130 possible sequences for a 100 amino acid protein) and extremely sparsely populated by functional proteins. Neither experimental,[4] nor natural[5] evolution can ever get close to sampling so many sequences. Of course, natural evolution samples variant sequences close to functional protein sequences and this is imitated in DE by mutagenising an already functional gene. Some calculations suggest it is entirely feasible that for all practical (i.e. functional and structural) purposes, protein sequence space has been fully explored during the course of evolution of life on Earth.[6]
The starting gene can be mutagenised by random point mutations (by chemical mutagens or error prone PCR)[7][8] and insertions and deletions (by transposons).[9] Gene recombination can be mimicked by DNA shuffling[10][11] of several sequences (usually of more than 70% homology) to jump into regions of sequence space between the shuffled parent genes. Finally, specific regions of a gene can be systematically randomised[12] for a more focused approach based on structure and function knowledge. Depending on the method, the library generated will vary in the proportion of functional variants it contains. Even if an organism is used to express the gene of interest, by mutagenising only that gene, the rest of the organism’s genome remains the same and can be ignored for the evolution experiment (to the extent of providing a constant genetic environment).
Detecting fitness differences
The majority of mutations are deleterious and so libraries of mutants tend to mostly have variants with reduced activity.[13] Therefore, a high-throughput assay is vital for measuring activity to find the rare variants with beneficial mutations that improve the desired properties. Two main categories of method exist for isolating functional variants. Selection systems directly couple protein function to survival of the gene, whereas screening systems individually assay each variant and allow a quantitative threshold to be set for sorting a variant or population of variants of a desired activity. Both selection and screening can be performed in living cells (in vivo evolution) or performed directly on the protein or RNA without any cells (in vitro evolution).[14][15]
During in vivo evolution, each cell (usually bacteria or yeast) is transformed with a plasmid containing a different member of the variant library. In this way, only the gene of interest differs between the cells, with all other genes being kept the same. The cells express the protein either in their cytoplasm or surface where its function can be tested. This format has the advantage of selecting for properties in a cellular environment, which is useful when the evolved protein or RNA is to be used in living organisms. When performed without cells, DE involves using in vitro transcription translation to produce proteins or RNA free in solution or compartmentalised in artificial microdroplets. This method has the benefits of being more versatile in the selection conditions (e.g. temperature, solvent), and can express proteins that would be toxic to cells. Furthermore, in vitro evolution experiments can generate far larger libraries (up to 1015) because the library DNA need not be inserted into cells (often a limiting step).
Selection
Selection for binding activity is conceptually simple. The target molecule is immobilised on a solid support, a library of variant proteins is flowed over it, poor binders are washed away, and the remaining bound variants recovered to isolate their genes.[16] Binding of an enzyme to immobilised covalent inhibitor has been also used as an attempt to isolate active catalysts. This approach, however, only selects for single catalytic turnover and is not a good model of substrate binding or true substrate reactivity. If an enzyme activity can be made necessary for cell survival, either by synthesizing a vital metabolite, or destroying a toxin, then cell survival is a function of enzyme activity.[17][18] Such systems are generally only limited in throughput by the transformation efficiency of cells. They are also less expensive and labour-intensive than screening, however they are typically difficult to engineer, prone to artefacts and give no information on the range of activities present in the library.
Screening
An alternative to selection is a screening system. Each variant gene is individually expressed and assayed to quantitatively measure the activity (most often by a colourgenic or fluorogenic product). The variants are then ranked and the experimenter decides which variants to use as temples for the next round of DE. Even the most high throughput assays usually have lower coverage than selection methods but give the advantage of producing detailed information on each one of the screened variants. This disaggregated data can also be used to characterise the distribution of activities in libraries which is not possible in simple selection systems. Screening systems, therefore, have advantages when it comes to experimentally characterising adaptive evolution and fitness landscapes.
Ensuring heredity

When functional proteins have been isolated, it is necessary that their genes are too, therefore a genotype-phenotype link is required.[19] This can be covalent, such as mRNA display where the mRNA gene is linked to the protein at the end of translation by puromycin.[20] Alternatively the protein and its gene can be co-localised by compartmentalisation in living cells[21] or emulsion droplets.[22] The gene sequences isolated are then amplified by PCR or by transformed host bacteria. Either the single best sequence, or a pool of sequences can be used as the template for the next round of mutagenesis. The repeated cycles of Diversification-Selection-Amplification generate protein variants adapted to the applied selection pressures.
Comparison to rational protein design
Advantages of directed evolution
Rational design of a protein relies on an in-depth knowledge of the protein structure, as well as its catalytic mechanism.[23][24] Specific changes are then made by site-directed mutagenesis in an attempt to change the function of the protein. A drawback of this is that even when the structure and mechanism of action of the protein are well known, the change due to mutation is still difficult to predict. Therefore, an advantage of DE is that there is no need to understand the mechanism of the desired activity or how mutations would affect it.[25]
Limitations of directed evolution
A restriction of directed evolution is that a high-throughput assay is required in order to measure the effects of a large number of different random mutations. This can require extensive research and development before it can be used for directed evolution. Additionally, such assays are often highly specific to monitoring a particular activity and so are not transferable to new DE experiments.[26]
Additionally, selecting for improvement in the assayed function simply generates improvements in the assayed function. To understand how these improvements are achieved, the properties of the evolving enzyme have to be measured. Improvement of the assayed activity can be due to improvements in enzyme catalytic activity or enzyme concentration. There is also no guarantee that improvement on one substrate will improve activity on another. This is particularly important when the desired activity cannot be directly screened or selected for and so a ‘proxy’ substrate is used. DE can lead to evolutionary specialisation to the proxy without improving the desired activity. Consequently, choosing appropriate screening or selection conditions is vital for successful DE.
Combinatorial approaches
Combined, 'semi-rational' approaches are being investigated to address the limitations of both rational design and directed evolution.[27][28] Beneficial mutations are rare, so large numbers of random mutants have to be screened to find improved variants. 'Focussed libraries' concentrate on randomising regions thought to be richer in beneficial mutations for the mutagenesis step of DE. A focussed library contains fewer variants than a traditional random mutagenesis library and so does not require such high-throughput screening.
Creating a focussed library requires some knowledge of which residues in the structure to mutate. For example, knowledge of the active site of an enzyme may allow just the residues known to interact with the substrate to be randomised.[29][30] Alternatively, knowledge of which protein regions are variable in nature can guide mutagenesis in just those regions.[31][32]
Uses
Directed evolution is frequently used for protein engineering as an alternative to rational design,[33] but can also be used to investigate fundamental questions of enzyme evolution.[34]
Protein engineering
As a protein engineering tool, DE has been most successful in three areas:
- Improving protein stability for biotechnological use at high temperatures or in harsh solvents.[35][36]
- Improving binding affinity of therapeutic antibodies (Affinity maturation)[37] and the activity of de novo designed enzymes.[38]
- Altering substrate specificity of existing enzymes,[39][40][41][42] (often for use in industry).[43]
Evolution studies
The study of natural evolution is traditionally based on extant organisms and their genes. However, research is fundamentally limited by the lack of fossils (and particularly the lack of ancient DNA sequences)[44][45] and incomplete knowledge of ancient environmental conditions. Directed evolution investigates evolution in a controlled system of genes for individual enzymes,[46][47][48] ribozymes[49] and replicators[50][51] (similar to experimental evolution of eukaryotes,[52][53] prokaryotes[54] and viruses[55]).
DE allows control of selection pressure, mutation rate and environment (both the abiotic environment such as temperature, and the biotic environment, such as other genes in the organism). Additionally, there is a complete record of all evolutionary intermediate genes. This allows for detailed measurements of evolutionary processes, for example epistasis, evolvability, adaptive constraint[56][57] fitness landscapes,[58] and neutral networks.[59]
See also
- Applications:
- Mutagenesis:
- Selection and screening:
References
- ↑ Stephen Lutz, Beyond directed evolution - semi-rational protein engineering and design, Curr Opin Biotechnol. 2010 December ; 21(6): 734–743.
- ↑ Voigt, CA; Kauffman, S; Wang, ZG (2000). "Rational evolutionary design: the theory of in vitro protein evolution.". Advances in protein chemistry. 55: 79–160. doi:10.1016/s0065-3233(01)55003-2. PMID 11050933.
- ↑ Dalby, PA (August 2011). "Strategy and success for the directed evolution of enzymes.". Current Opinion in Structural Biology. 21 (4): 473–80. doi:10.1016/j.sbi.2011.05.003. PMID 21684150.
- ↑ Lipovsek, D; Plückthun, A (July 2004). "In-vitro protein evolution by ribosome display and mRNA display.". Journal of immunological methods. 290 (1-2): 51–67. doi:10.1016/j.jim.2004.04.008. PMID 15261571.
- ↑ Dryden, DT; Thomson, AR; White, JH (6 August 2008). "How much of protein sequence space has been explored by life on Earth?". Journal of the Royal Society, Interface / the Royal Society. 5 (25): 953–6. doi:10.1098/rsif.2008.0085. PMID 18426772.
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2459213/
- ↑ Kuchner, O; Arnold, FH (December 1997). "Directed evolution of enzyme catalysts.". Trends in Biotechnology. 15 (12): 523–30. doi:10.1016/s0167-7799(97)01138-4. PMID 9418307.
- ↑ Sen, S; Venkata Dasu, V; Mandal, B (December 2007). "Developments in directed evolution for improving enzyme functions.". Applied biochemistry and biotechnology. 143 (3): 212–23. doi:10.1007/s12010-007-8003-4. PMID 18057449.
- ↑ Jones, DD (16 May 2005). "Triplet nucleotide removal at random positions in a target gene: the tolerance of TEM-1 beta-lactamase to an amino acid deletion.". Nucleic Acids Research. 33 (9): e80. doi:10.1093/nar/gni077. PMID 15897323.
- ↑ Stemmer, WP (4 August 1994). "Rapid evolution of a protein in vitro by DNA shuffling.". Nature. 370 (6488): 389–91. doi:10.1038/370389a0. PMID 8047147.
- ↑ Crameri, A; Raillard, SA; Bermudez, E; Stemmer, WP (15 January 1998). "DNA shuffling of a family of genes from diverse species accelerates directed evolution.". Nature. 391 (6664): 288–91. doi:10.1038/34663. PMID 9440693.
- ↑ Reetz, MT; Carballeira, JD (2007). "Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes.". Nature protocols. 2 (4): 891–903. doi:10.1038/nprot.2007.72. PMID 17446890.
- ↑ Hartl, DL (October 2014). "What can we learn from fitness landscapes?". Current opinion in microbiology. 21C: 51–57. doi:10.1016/j.mib.2014.08.001. PMID 25444121.
- ↑ Badran, AH; Liu, DR (7 November 2014). "In vivo continuous directed evolution.". Current Opinion in Chemical Biology. 24C: 1–10. doi:10.1016/j.cbpa.2014.09.040. PMID 25461718.
- ↑ Kumar, A; Singh, S (December 2013). "Directed evolution: tailoring biocatalysts for industrial applications.". Critical reviews in biotechnology. 33 (4): 365–78. doi:10.3109/07388551.2012.716810. PMID 22985113.
- ↑ Willats, WG (December 2002). "Phage display: practicalities and prospects.". Plant molecular biology. 50 (6): 837–54. PMID 12516857.
- ↑ Leemhuis, H; Stein, V; Griffiths, AD; Hollfelder, F (August 2005). "New genotype-phenotype linkages for directed evolution of functional proteins.". Current Opinion in Structural Biology. 15 (4): 472–8. doi:10.1016/j.sbi.2005.07.006. PMID 16043338.
- ↑ Verhoeven, KD; Altstadt, OC; Savinov, SN (March 2012). "Intracellular detection and evolution of site-specific proteases using a genetic selection system.". Applied biochemistry and biotechnology. 166 (5): 1340–54. doi:10.1007/s12010-011-9522-6. PMID 22270548.
- ↑ Leemhuis, H; Stein, V; Griffiths, AD; Hollfelder, F (August 2005). "New genotype-phenotype linkages for directed evolution of functional proteins.". Current Opinion in Structural Biology. 15 (4): 472–8. doi:10.1016/j.sbi.2005.07.006. PMID 16043338.
- ↑ Lipovsek, D; Plückthun, A (July 2004). "In-vitro protein evolution by ribosome display and mRNA display.". Journal of immunological methods. 290 (1-2): 51–67. doi:10.1016/j.jim.2004.04.008. PMID 15261571.
- ↑ Nguyen, AW; Daugherty, PS (March 2005). "Evolutionary optimization of fluorescent proteins for intracellular FRET.". Nature Biotechnology. 23 (3): 355–60. doi:10.1038/nbt1066. PMID 15696158.
- ↑ Schaerli, Y; Hollfelder, F (December 2009). "The potential of microfluidic water-in-oil droplets in experimental biology.". Molecular bioSystems. 5 (12): 1392–404. doi:10.1039/b907578j. PMID 20023716.
- ↑ Marshall, SA; Lazar, GA; Chirino, AJ; Desjarlais, JR (1 March 2003). "Rational design and engineering of therapeutic proteins.". Drug Discovery Today. 8 (5): 212–21. doi:10.1016/s1359-6446(03)02610-2. PMID 12634013.
- ↑ Wilson, CJ (27 October 2014). "Rational protein design: developing next-generation biological therapeutics and nanobiotechnological tools.". Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. doi:10.1002/wnan.1310. PMID 25348497.
- ↑ Giger, L; Caner, S; Obexer, R; Kast, P; Baker, D; Ban, N; Hilvert, D (August 2013). "Evolution of a designed retro-aldolase leads to complete active site remodeling.". Nature Chemical Biology. 9 (8): 494–8. doi:10.1038/nchembio.1276. PMID 23748672.
- ↑ Bornscheuer, UT; Pohl, M (April 2001). "Improved biocatalysts by directed evolution and rational protein design.". Current Opinion in Chemical Biology. 5 (2): 137–43. doi:10.1016/s1367-5931(00)00182-4. PMID 11282339.
- ↑ Lutz, S (December 2010). "Beyond directed evolution--semi-rational protein engineering and design.". Current opinion in biotechnology. 21 (6): 734–43. doi:10.1016/j.copbio.2010.08.011. PMID 20869867.
- ↑ Goldsmith, M; Tawfik, DS (August 2012). "Directed enzyme evolution: beyond the low-hanging fruit.". Current Opinion in Structural Biology. 22 (4): 406–12. doi:10.1016/j.sbi.2012.03.010. PMID 22579412.
- ↑ Chen, MM; Snow, CD; Vizcarra, CL; Mayo, SL; Arnold, FH (April 2012). "Comparison of random mutagenesis and semi-rational designed libraries for improved cytochrome P450 BM3-catalyzed hydroxylation of small alkanes.". Protein engineering, design & selection : PEDS. 25 (4): 171–8. doi:10.1093/protein/gzs004. PMID 22334757.
- ↑ Acevedo-Rocha, CG; Hoebenreich, S; Reetz, MT (2014). "Iterative saturation mutagenesis: a powerful approach to engineer proteins by systematically simulating Darwinian evolution.". Methods in molecular biology (Clifton, N.J.). 1179: 103–28. doi:10.1007/978-1-4939-1053-3_7. PMID 25055773.
- ↑ Jochens, H; Bornscheuer, UT (3 September 2010). "Natural diversity to guide focused directed evolution.". Chembiochem : a European journal of chemical biology. 11 (13): 1861–6. doi:10.1002/cbic.201000284. PMID 20680978.
- ↑ Jochens, H; Aerts, D; Bornscheuer, UT (December 2010). "Thermostabilization of an esterase by alignment-guided focussed directed evolution.". Protein engineering, design & selection : PEDS. 23 (12): 903–9. doi:10.1093/protein/gzq071. PMID 20947674.
- ↑ Turner, NJ (August 2009). "Directed evolution drives the next generation of biocatalysts.". Nature Chemical Biology. 5 (8): 567–73. doi:10.1038/nchembio.203. PMID 19620998.
- ↑ Romero, PA; Arnold, FH (December 2009). "Exploring protein fitness landscapes by directed evolution.". Nature reviews. Molecular cell biology. 10 (12): 866–76. doi:10.1038/nrm2805. PMC 2997618
 . PMID 19935669.
. PMID 19935669. - ↑ Gatti-Lafranconi, P; Natalello, A; Rehm, S; Doglia, SM; Pleiss, J; Lotti, M (8 January 2010). "Evolution of stability in a cold-active enzyme elicits specificity relaxation and highlights substrate-related effects on temperature adaptation.". Journal of Molecular Biology. 395 (1): 155–66. doi:10.1016/j.jmb.2009.10.026. PMID 19850050.
- ↑ Zhao, H; Arnold, FH (January 1999). "Directed evolution converts subtilisin E into a functional equivalent of thermitase.". Protein engineering. 12 (1): 47–53. doi:10.1093/protein/12.1.47. PMID 10065710.
- ↑ Hawkins, RE; Russell, SJ; Winter, G (5 August 1992). "Selection of phage antibodies by binding affinity. Mimicking affinity maturation.". Journal of Molecular Biology. 226 (3): 889–96. doi:10.1016/0022-2836(92)90639-2. PMID 1507232.
- ↑ Giger, L; Caner, S; Obexer, R; Kast, P; Baker, D; Ban, N; Hilvert, D (August 2013). "Evolution of a designed retro-aldolase leads to complete active site remodeling.". Nature Chemical Biology. 9 (8): 494–8. doi:10.1038/nchembio.1276. PMID 23748672.
- ↑ Shaikh, FA; Withers, SG (April 2008). "Teaching old enzymes new tricks: engineering and evolution of glycosidases and glycosyl transferases for improved glycoside synthesis.". Biochemistry and cell biology = Biochimie et biologie cellulaire. 86 (2): 169–77. doi:10.1139/o07-149. PMID 18443630.
- ↑ Cheriyan, M; Walters, MJ; Kang, BD; Anzaldi, LL; Toone, EJ; Fierke, CA (1 November 2011). "Directed evolution of a pyruvate aldolase to recognize a long chain acyl substrate.". Bioorganic & Medicinal Chemistry. 19 (21): 6447–53. doi:10.1016/j.bmc.2011.08.056. PMID 21944547.
- ↑ MacBeath, G; Kast, P; Hilvert, D (20 March 1998). "Redesigning enzyme topology by directed evolution.". Science. 279 (5358): 1958–61. doi:10.1126/science.279.5358.1958. PMID 9506949.
- ↑ Toscano, MD; Woycechowsky, KJ; Hilvert, D (2007). "Minimalist active-site redesign: teaching old enzymes new tricks.". Angewandte Chemie International Edition in English. 46 (18): 3212–36. doi:10.1002/anie.200604205. PMID 17450624.
- ↑ Turner, NJ (August 2009). "Directed evolution drives the next generation of biocatalysts.". Nature Chemical Biology. 5 (8): 567–73. doi:10.1038/nchembio.203. PMID 19620998.
- ↑ Pääbo, S; Poinar, H; Serre, D; Jaenicke-Despres, V; Hebler, J; Rohland, N; Kuch, M; Krause, J; Vigilant, L; Hofreiter, M (2004). "Genetic analyses from ancient DNA.". Annual Review of Genetics. 38 (1): 645–79. doi:10.1146/annurev.genet.37.110801.143214. PMID 15568989.
- ↑ Höss, M; Jaruga, P; Zastawny, TH; Dizdaroglu, M; Pääbo, S (1 April 1996). "DNA damage and DNA sequence retrieval from ancient tissues.". Nucleic Acids Research. 24 (7): 1304–7. doi:10.1093/nar/24.7.1304. PMID 8614634.
- ↑ Bloom, JD; Arnold, FH (16 June 2009). "In the light of directed evolution: pathways of adaptive protein evolution.". Proceedings of the National Academy of Sciences of the United States of America. 106 Suppl 1 (Supplement_1): 9995–10000. doi:10.1073/pnas.0901522106. PMID 19528653.
- ↑ Moses, AM; Davidson, AR (17 May 2011). "In vitro evolution goes deep.". Proceedings of the National Academy of Sciences of the United States of America. 108 (20): 8071–2. doi:10.1073/pnas.1104843108. PMID 21551096.
- ↑ Goldsmith, M; Tawfik, DS (August 2012). "Directed enzyme evolution: beyond the low-hanging fruit.". Current Opinion in Structural Biology. 22 (4): 406–12. doi:10.1016/j.sbi.2012.03.010. PMID 22579412.
- ↑ Salehi-Ashtiani, K; Szostak, JW (1 November 2001). "In vitro evolution suggests multiple origins for the hammerhead ribozyme.". Nature. 414 (6859): 82–4. doi:10.1038/35102081. PMID 11689947.
- ↑ Sumper, M; Luce, R (January 1975). "Evidence for de novo production of self-replicating and environmentally adapted RNA structures by bacteriophage Qbeta replicase.". Proceedings of the National Academy of Sciences of the United States of America. 72 (1): 162–6. doi:10.1073/pnas.72.1.162. PMC 432262
 . PMID 1054493.
. PMID 1054493. - ↑ Mills, DR; Peterson, RL; Spiegelman, S (July 1967). "An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule.". Proceedings of the National Academy of Sciences of the United States of America. 58 (1): 217–24. doi:10.1073/pnas.58.1.217. PMC 335620
 . PMID 5231602.
. PMID 5231602. - ↑ Marden, JH; Wolf, MR; Weber, KE (November 1997). "Aerial performance of Drosophila melanogaster from populations selected for upwind flight ability.". The Journal of Experimental Biology. 200 (Pt 21): 2747–55. PMID 9418031.
- ↑ Ratcliff, WC; Denison, RF; Borrello, M; Travisano, M (31 January 2012). "Experimental evolution of multicellularity.". Proceedings of the National Academy of Sciences of the United States of America. 109 (5): 1595–600. doi:10.1073/pnas.1115323109. PMID 22307617.
- ↑ Barrick, JE; Yu, DS; Yoon, SH; Jeong, H; Oh, TK; Schneider, D; Lenski, RE; Kim, JF (29 October 2009). "Genome evolution and adaptation in a long-term experiment with Escherichia coli.". Nature. 461 (7268): 1243–7. doi:10.1038/nature08480. PMID 19838166.
- ↑ Heineman, RH; Molineux, IJ; Bull, JJ (August 2005). "Evolutionary robustness of an optimal phenotype: re-evolution of lysis in a bacteriophage deleted for its lysin gene.". Journal of Molecular Evolution. 61 (2): 181–91. doi:10.1007/s00239-004-0304-4. PMID 16096681.
- ↑ Steinberg, Barrett; Ostermeier, Marc (2016-01-01). "Environmental changes bridge evolutionary valleys". Science Advances. 2 (1): e1500921. doi:10.1126/sciadv.1500921. ISSN 2375-2548. PMC 4737206
 . PMID 26844293.
. PMID 26844293. - ↑ Arnold, FH; Wintrode, PL; Miyazaki, K; Gershenson, A (February 2001). "How enzymes adapt: lessons from directed evolution.". Trends in Biochemical Sciences. 26 (2): 100–6. doi:10.1016/s0968-0004(00)01755-2. PMID 11166567.
- ↑ Aita, T; Hamamatsu, N; Nomiya, Y; Uchiyama, H; Shibanaka, Y; Husimi, Y (5 July 2002). "Surveying a local fitness landscape of a protein with epistatic sites for the study of directed evolution.". Biopolymers. 64 (2): 95–105. doi:10.1002/bip.10126. PMID 11979520.
- ↑ Bloom, JD; Raval, A; Wilke, CO (January 2007). "Thermodynamics of neutral protein evolution.". Genetics. 175 (1): 255–66. doi:10.1534/genetics.106.061754. PMID 17110496.
External links
- Research groups
- The Dan Tawfik Research Group
- The Ulrich Schwaneberg Research Group
- The Frances Arnold Research Group
- The Huimin Zhao Research Group
- The Manfred Reetz Research Group
- The Donald Hilvert Group
- The Darren Hart Research Group
- The David Liu Research Group
- The Douglas Clark Research Group
- The Paul Dalby Research Group
- SeSaM-Biotech - Directed Evolution
- Prof. Reetz explains the principle of Directed Evolution
- Codexis, Inc.