Indolamines
Indolamines are a family of neurotransmitters that share a common molecular structure (namely, indolamine). Indolamines are a classification of monoamine neurotransmitter, along with catecholamines and ethylamine. A common example of an indolamine is the tryptophan derivative serotonin, a neurotransmitter involved in mood and sleep.[1] Another example of an indolamine is melatonin, which regulates the sleep-wake cycle (circadian rhythm) in humans.
In biochemistry, indolamines are substituted indole compounds that contain an amino group. Examples of indoleamines include the lysergamides.
Synthesis
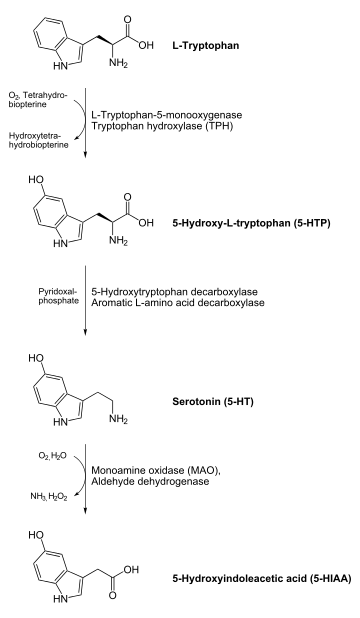
In humans, neurotransmitters in the indolamine family are believed to be produced in the pineal gland. Indolamines are biologically synthesized from the essential amino acid tryptophan. Tryptophan is synthesized into serotonin through the addition of a hydroxyl group by the enzyme tryptophan hydroxylase and the subsequent removal of the carboxyl group by the enzyme 5-HTP decarboxylase.[2]
See also
References
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2077351/
- ↑ Carlson, Neil R. Physiology of Behavior. 11th ed. Vol. 1. N.p.: Pearson Education, n.d. Print.