Lipocalin 2
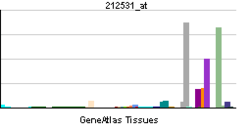
| View/Edit Human | View/Edit Mouse |
Lipocalin-2 (LCN2), also known as oncogene 24p3 or neutrophil gelatinase-associated lipocalin (NGAL), is a protein that in humans is encoded by the LCN2 gene.[3][4][5] NGAL is involved in innate immunity by sequestrating iron that in turn limits bacterial growth.[6] It is expressed in neutrophils and in low levels in the kidney, prostate, and epithelia of the respiratory and alimentary tracts.[5][7] NGAL is used as a biomarker of kidney injury.[8]
Function
The binding of NGAL to bacterial siderophores is important in the innate immune response to bacterial infection. Upon encountering invading bacteria the toll-like receptors on immune cells stimulate the synthesis and secretion of NGAL. Secreted NGAL then limits bacterial growth by sequestering iron-containing siderophores.[9][10] Lipocalin-2 binds, next to bacterial siderophores, also to the mammalian siderophore 2,5-dihydroxybenzoic acid (2,5-DHBA). This complex ensures that excess free iron does not accumulate in the cytoplasm. Mammalian cells lacking 2,5-DHBA accumulate abnormal intracellular levels of iron leading to high levels of reactive oxygen species.[11] Lipocalin-2 also functions as a growth factor.[10]
Clinical significance
In the case of acute kidney injury (AKI), NGAL is secreted in high levels into the blood and urine within 2 hours of injury.[12] Because NGAL is protease resistant and small, the protein is easily excreted and detected in the urine.[13] NGAL levels in patients with AKI have been associated with the severity of their prognosis and can be used as a biomarker for AKI[12] NGAL can also be used as an early diagnosis for procedures such as chronic kidney disease, contrast induced nephropathy, and kidney transplant.[14]
Kidney health is most frequently measured by serum creatinine. Serum creatinine is a marker of kidney function, whereas NGAL is a marker of kidney injury.[15] NGAL levels are a more precise and sensitive marker for diagnosing AKI than serum creatinine levels. In fact, the increase in urinary excretion of NGAL has been proven to be due to tubular alterations that take place before any damage can be detected by other methods [16] Therefore, monitoring NGAL levels reduces delayed AKI diagnosis and treatment.[17] Using a more sensitive and specific marker allows for earlier diagnosis, correct responses to AKI, and reduced risk of morbidity and mortality.[18]
The NGAL level measured in an individual is proportional to the severity of the AKI.[18] Individuals positive for NGAL tend to have higher incidence of renal replacement therapy and have higher rates of in-hospital mortality, both in the presence and the absence of serum creatinine.[18] Therefore, an individual may have AKI without the presence of serum creatinine.
The ability to diagnose AKI before acute kidney failure is financially beneficial and favorable for preventative health measures. More than 10% of people in the United States will develop some kind of chronic kidney disease (CKD), with higher incidences for individuals that suffer from obesity, elevated cholesterol, and a family history of CKD. There is no point of return once there is a significant injury to the kidney; therefore, early diagnosis of kidney injury is important for preventing AKI. Using NGAL as a biomarker can lower hospital costs because less patients will reach a critical stage in kidney injury. Ultimately, diagnosis of AKI with NGAL can reduce the time a patient stays in a hospital. For example, the early diagnosis of AKI with NGAL as a biomarker can help a patient avoid kidney dialysis.
NGAL may be used to detect and monitor the potential additive effects of different comorbid factors (e.g. hypertension and hyperglycemia), which makes it a very useful marker, since there is an increasing number of patients with comorbidities.[16]
Laboratory measurement
Renal expression of NGAL increases in the kidneys after injury for a variety of reasons. The level of NGAL in the urine and plasma increases within 2 hours of kidney injury. It is possible to measure NGAL in serum or urine in the range of 25 to 5,000 ng/mL by current laboratory tests.[19] Low levels for NGAL have been considered to be 20 ng/mL, medium levels 200 ng/mL, and high levels 1200 ng/mL.[19]
A study on children with pediatric cardiopulmonary bypass operations showed that urinary NGAL concentrations above 50 ng/mL 2 hours after surgery is indicative of serum creatinine levels 50% over basal values. Normally, children tend to have almost undetectable levels of NGAL.[20] Therefore, studies that include children are considered to be “pure.” Adult patients presenting for cardiopulmonary bypass surgery are not considered to be “pure” in NGAL studies because adults often have other disorders such as inflammatory conditions, which can cause slight increases in NGAL.
AKI studies investigating the use of NGAL as a biomarker often compare serum creatinine and NGAL production. Unfortunately, serum creatinine production is variable and can reflect hemodynamic variation in the glomerular filtration rate formerly known as prerenal azotemia; therefore, the comparison is not always reliable because creatinine and NGAL measure different components of renal (dys)function.[13] The demonstration that NGAL does not rise in the setting of transient changes in creatinine can help clinicians determine whether changes in creatinine reflect kidney damage or rather only non specific or mild functional changes in kidney function.
In summary, hundreds of clinical studies demonstrate that NGAL measures kidney injury.
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N (May 1993). "Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase". J. Biol. Chem. 268 (14): 10425–32. PMID 7683678.
- ↑ Chan P, Simon-Chazottes D, Mattei MG, Guenet JL, Salier JP (September 1994). "Comparative mapping of lipocalin genes in human and mouse: the four genes for complement C8 gamma chain, prostaglandin-D-synthase, oncogene-24p3, and progestagen-associated endometrial protein map to HSA9 and MMU2". Genomics. 23 (1): 145–50. doi:10.1006/geno.1994.1470. PMID 7829063.
- 1 2 Cowland JB, Borregaard N (October 1997). "Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans". Genomics. 45 (1): 17–23. doi:10.1006/geno.1997.4896. PMID 9339356.
- ↑ Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J (November 2002). "An iron delivery pathway mediated by a lipocalin". Mol. Cell. 10 (5): 1045–56. doi:10.1016/S1097-2765(02)00710-4. PMID 12453413.
- ↑ Friedl A, Stoesz SP, Buckley P, Gould MN (July 1999). "Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression". Histochem. J. 31 (7): 433–41. doi:10.1023/A:1003708808934. PMID 10475571.
- ↑ Devarajan P (June 2010). "Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury". Nephrology (Carlton). 15 (4): 419–28. doi:10.1111/j.1440-1797.2010.01317.x. PMID 20609093.
- ↑ Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A (December 2004). "Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron". Nature. 432 (7019): 917–21. doi:10.1038/nature03104. PMID 15531878.
- 1 2 Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J (February 2007). "Dual action of neutrophil gelatinase-associated lipocalin". J. Am. Soc. Nephrol. 18 (2): 407–13. doi:10.1681/ASN.2006080882. PMID 17229907.
- ↑ Devireddy LR, Hart DO, Goetz DH, Green MR (June 2010). "A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production". Cell. 141 (6): 1006–17. doi:10.1016/j.cell.2010.04.040. PMC 2910436
 . PMID 20550936.
. PMID 20550936. - 1 2 Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P (May 2008). "Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study". Clin J Am Soc Nephrol. 3 (3): 665–73. doi:10.2215/CJN.04010907. PMC 2386703
 . PMID 18337554.
. PMID 18337554. - 1 2 Uttenthal LO (April 2007). "NGAL: How Useful Is the New Marker of Kidney Damage?" (PDF). CLI.
- ↑ Goldstein SL (2011). "Acute kidney injury biomarkers: renal angina and the need for a renal troponin I". BMC Med. 9: 135. doi:10.1186/1741-7015-9-135. PMC 3287120
 . PMID 22189039.
. PMID 22189039. - ↑ Han WK, Wagener G, Zhu Y, Wang S, Lee HT (May 2009). "Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery". Clin J Am Soc Nephrol. 4 (5): 873–82. doi:10.2215/CJN.04810908. PMC 2676184
 . PMID 19406962.
. PMID 19406962. - 1 2 Blázquez-Medela AM, García-Sánchez O, Blanco-Gozalo V, Quiros Y, Montero MJ, Martínez-Salgado C, López-Novoa JM, López-Hernández FJ (August 2014). "Hypertension and Hyperglycemia Synergize to Cause Incipient Renal Tubular Alterations Resulting in Increased NGAL Urinary Excretion in Rats". PLoS ONE. 9 (8): e105988. doi:10.1371/journal.pone.0105988. PMC 4141836
 . PMID 25148248.
. PMID 25148248. - ↑ Lerma EV (2012). "Novel Biomarkers of Renal Function". Medscape.
- 1 2 3 Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR (April 2011). "The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies". J. Am. Coll. Cardiol. 57 (17): 1752–61. doi:10.1016/j.jacc.2010.11.051. PMID 21511111.
- 1 2 Lippi G, Aloe R, Storelli A, Cervellin G, Trenti T (2012). "Evaluation of NGAL Test™, a fully-automated neutrophil gelatinase-associated lipocalin (NGAL) immunoassay on Beckman Coulter AU 5822". Clin. Chem. Lab. Med. 50 (9): 1581–4. doi:10.1515/cclm.2011.839. PMID 22962213.
- ↑ Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P (June 2011). "Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass". J. Pediatr. 158 (6): 1009–1015.e1. doi:10.1016/j.jpeds.2010.12.057. PMID 21300375.