ACP1
Low molecular weight phosphotyrosine protein phosphatase is an enzyme that in humans is encoded by the ACP1 gene.
The product of this gene belongs to the phosphotyrosine protein phosphatase family of proteins. It functions as an acid phosphatase and a protein tyrosine phosphatase by hydrolyzing protein tyrosine phosphate to protein tyrosine and orthophosphate. This enzyme also hydrolyzes orthophosphoric monoesters to alcohol and orthophosphate. This gene is genetically polymorphic, and three common alleles segregating at the corresponding locus give rise to six phenotypes. Each allele appears to encode at least two electrophoretically different isozymes, Bf and Bs, which are produced in allele-specific ratios. Three transcript variants encoding distinct isoforms have been identified for this gene.[3]
Interactions
ACP1 has been shown to interact with EPH receptor A2[4] and EPH receptor B1.[5]
References
External links
Further reading
- Junien C, Kaplan JC, Bernheim A, Berger R (1979). "Regional assignment of red cell acid phosphatase locus to band 2p25.". Hum. Genet. 48 (1): 17–21. doi:10.1007/BF00273269. PMID 457131.
- Sensabaugh GF, Golden VL (1979). "Phenotype dependence in the inhibition of red cell acid phosphatase (ACP) by folates.". American Journal of Human Genetics. 30 (5): 553–60. PMC 1685602
 . PMID 736044.
. PMID 736044.
- Shekels LL, Smith AJ, Van Etten RL, Bernlohr DA (1993). "Identification of the adipocyte acid phosphatase as a PAO-sensitive tyrosyl phosphatase.". Protein Sci. 1 (6): 710–21. doi:10.1002/pro.5560010603. PMC 2142247
 . PMID 1304913.
. PMID 1304913.
- Wo YY, McCormack AL, Shabanowitz J, et al. (1992). "Sequencing, cloning, and expression of human red cell-type acid phosphatase, a cytoplasmic phosphotyrosyl protein phosphatase.". J. Biol. Chem. 267 (15): 10856–65. PMID 1587862.
- Dissing J, Johnsen AH (1992). "Human red cell acid phosphatase (ACP1): the primary structure of the two pairs of isozymes encoded by the ACP1*A and ACP1*C alleles.". Biochim. Biophys. Acta. 1121 (3): 261–8. doi:10.1016/0167-4838(92)90155-7. PMID 1627603.
- Dissing J, Johnsen AH, Sensabaugh GF (1991). "Human red cell acid phosphatase (ACP1). The amino acid sequence of the two isozymes Bf and Bs encoded by the ACP1*B allele.". J. Biol. Chem. 266 (31): 20619–25. PMID 1939112.
- Wakita Y, Narahara K, Takahashi Y, et al. (1986). "Duplication of 2p25: confirmation of the assignment of soluble acid phosphatase (ACP1) locus to 2p25.". Hum. Genet. 71 (3): 259–60. doi:10.1007/BF00284586. PMID 4065897.
- Blake NM, Kirk RL, Barnes KR, Thompson JM (1974). "Expression of human red cell acid phosphatase activity in placenta and other tissues.". Jinrui Idengaku Zasshi. 18 (1): 10–23. PMID 4356849.
- Sensabaugh GF, Lazaruk KA (1993). "A TaqI site identifies the *A allele at the ACP1 locus.". Hum. Mol. Genet. 2 (7): 1079. doi:10.1093/hmg/2.7.1079-a. PMID 8364553.
- Bryson GL, Massa H, Trask BJ, Van Etten RL (1996). "Gene structure, sequence, and chromosomal localization of the human red cell-type low-molecular-weight acid phosphotyrosyl phosphatase gene, ACP1.". Genomics. 30 (2): 133–40. doi:10.1006/geno.1995.9893. PMID 8586411.
- Tailor P, Gilman J, Williams S, et al. (1997). "Regulation of the low molecular weight phosphotyrosine phosphatase by phosphorylation at tyrosines 131 and 132.". J. Biol. Chem. 272 (9): 5371–4. doi:10.1074/jbc.272.9.5371. PMID 9038134.
- Stein E, Lane AA, Cerretti DP, et al. (1998). "Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses.". Genes Dev. 12 (5): 667–78. doi:10.1101/gad.12.5.667. PMC 316584
 . PMID 9499402.
. PMID 9499402.
- Modesti A, Marzocchini R, Raugei G, et al. (1998). "Cloning, expression and characterisation of a new human low Mr phosphotyrosine protein phosphatase originating by alternative splicing.". FEBS Lett. 431 (1): 111–5. doi:10.1016/S0014-5793(98)00732-7. PMID 9684876.
- Zhang M, Stauffacher CV, Lin D, Van Etten RL (1998). "Crystal structure of a human low molecular weight phosphotyrosyl phosphatase. Implications for substrate specificity.". J. Biol. Chem. 273 (34): 21714–20. doi:10.1074/jbc.273.34.21714. PMID 9705307.
- Tailor P, Gilman J, Williams S, Mustelin T (1999). "A novel isoform of the low molecular weight phosphotyrosine phosphatase, LMPTP-C, arising from alternative mRNA splicing.". Eur. J. Biochem. 262 (2): 277–82. doi:10.1046/j.1432-1327.1999.00353.x. PMID 10336608.
- Huang L, Sankar S, Lin C, et al. (2000). "HCPTPA, a protein tyrosine phosphatase that regulates vascular endothelial growth factor receptor-mediated signal transduction and biological activity.". J. Biol. Chem. 274 (53): 38183–8. doi:10.1074/jbc.274.53.38183. PMID 10608891.
- Nicolas G, Fournier CM, Galand C, et al. (2002). "Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain.". Mol. Cell. Biol. 22 (10): 3527–36. doi:10.1128/MCB.22.10.3527-3536.2002. PMC 133798
 . PMID 11971983.
. PMID 11971983.
- Bottini N, Stefanini L, Williams S, et al. (2002). "Activation of ZAP-70 through specific dephosphorylation at the inhibitory Tyr-292 by the low molecular weight phosphotyrosine phosphatase (LMPTP).". J. Biol. Chem. 277 (27): 24220–4. doi:10.1074/jbc.M202885200. PMID 11976341.
- Bottini N, Ammendola M, Gloria-Bottini F (2002). "ACP1 is associated with allergy.". Allergy. 57 (7): 651–2. doi:10.1034/j.1398-9995.2002.23722.x. PMID 12100313.
- Kikawa KD, Vidale DR, Van Etten RL, Kinch MS (2002). "Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation.". J. Biol. Chem. 277 (42): 39274–9. doi:10.1074/jbc.M207127200. PMID 12167657.
PDB gallery |
|---|
|
-
1bvh: SOLUTION STRUCTURE OF A LOW MOLECULAR WEIGHT PROTEIN TYROSINE PHOSPHATASE
-
1c0e: Active Site S19A Mutant of Bovine Heart Phosphotyrosyl Phosphatase
-
1dg9: CRYSTAL STRUCTURE OF BOVINE LOW MOLECULAR WEIGHT PTPASE COMPLEXED WITH HEPES
-
1phr: THE CRYSTAL STRUCTURE OF A LOW MOLECULAR PHOSPHOTYROSINE PROTEIN PHOSPHATASE
-
1pnt: CRYSTAL STRUCTURE OF BOVINE HEART PHOSPHOTYROSYL PHOSPHATASE AT 2.2 ANGSTROMS RESOLUTION
-
1xww: Crystal Structure of Human B-form Low Molecular Weight Phosphotyrosyl Phosphatase at 1.6 Angstrom Resolution
-
1z12: Crystal Structure of Bovine Low Molecular Weight PTPase Complexed with Vanadate
-
1z13: Crystal Structure of Bovine Low Molecular Weight PTPase Complexed with Molybdate
-
5pnt: CRYSTAL STRUCTURE OF A HUMAN LOW MOLECULAR WEIGHT PHOSPHOTYROSYL PHOSPHATASE. IMPLICATIONS FOR SUBSTRATE SPECIFICITY
|


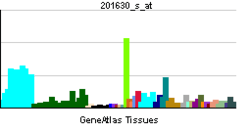
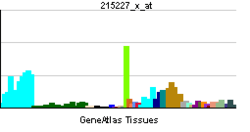
 . PMID 9499402.
. PMID 9499402. . PMID 736044.
. PMID 736044. . PMID 1304913.
. PMID 1304913. . PMID 9499402.
. PMID 9499402. . PMID 11971983.
. PMID 11971983.