Lac repressor
The lac repressor is a DNA-binding protein which inhibits the expression of genes coding for proteins involved in the metabolism of lactose in bacteria. These genes are repressed when lactose is not available to the cell, ensuring that the bacterium only invests energy in the production of machinery necessary for uptake and utilization of lactose when lactose is present. When lactose becomes available, it is converted into allolactose, which inhibits the lac repressor's DNA binding ability. Loss of DNA binding by the lac repressor is required for transcriptional activation of the operon.
Function
The lac repressor (LacI) operates by a helix-turn-helix motif in its DNA binding domain binding base-specifically to the major groove of the operator region of the lac operon, with base contacts also made by residues of symmetry-related alpha helices, the "hinge" helices, which bind deeply in the minor groove.[1] This DNA binding causes the specific affinity of RNA polymerase for the promoter sequence to increase sufficiently that it cannot escape the promoter region and enter elongation, and so prevents transcription of the mRNA coding for the Lac proteins.[2] When lactose is present, allolactose binds to the lac repressor, causing an allosteric change in its shape. In its changed state, the lac repressor is unable to bind tightly to its cognate operator. This effect is referred to as induction, because it induces, rather than represses, expression of the metabolic genes. In vitro, Isopropyl β-D-1-thiogalactopyranoside (IPTG) is a commonly used allolactose mimic which can be used to induce transcription of genes being regulated by lac repressor.
Structure
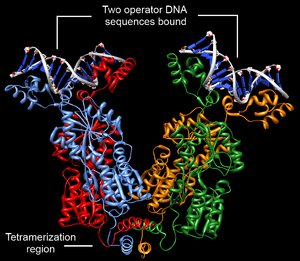
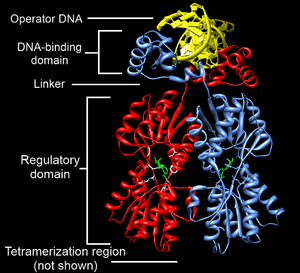
Structurally, the lac repressor protein is a homo-tetramer. The tetramer contains two DNA binding subunits composed of two monomers each (sometimes called "dimeric lac repressor"). These subunits dimerize to form a tetramer capable of binding two operator sequences. Each monomer [3][4][5] consists of four distinct regions:
- an N-terminal DNA-binding domain (in which two LacI proteins bind a single operator site)
- a regulatory domain (sometimes called the core domain, which binds allolactose, an allosteric effector molecule)
- a linker that connects the DNA-binding domain with the core domain (sometimes called the hinge helix, which is important for allosteric communication[5])
- a C-terminal tetramerization region (which joins four monomers in an alpha-helix bundle)
DNA binding occurs via an N-terminal helix-turn-helix structural motif and is targeted to one of several operator DNA sequences (known as O1, O2 and O3). The O1 operator sequence slightly overlaps with the promoter, which increases the affinity of RNA polymerase for the promoter sequence such that it cannot enter elongation and remains in Abortive initiation. Additionally, because each tetramer contains two DNA-binding subunits, binding of multiple operator sequences by a single tetramer induces DNA looping.[6]
Discovery
The lac repressor was first isolated by Walter Gilbert and Benno Müller-Hill in 1966.[7] They were able to show, in vitro, that the protein bound to DNA containing the lac operon, and released the DNA when IPTG was added. (IPTG is an allolactose analog.) They were also able to isolate the portion of DNA bound by the protein by using the enzyme deoxyribonuclease, which breaks down DNA. After treatment of the repressor-DNA complex, some DNA remained, suggesting that it had been masked by the repressor. This was later confirmed.
These experiments confirmed the mechanism of the lac operon, earlier proposed by Jacques Monod and Francois Jacob.
See also
References
- ↑ Schumacher, M. A.; Choi, K. Y.; Zalkin, H; Brennan, R. G. (1994). "Crystal structure of LacI member, PurR, bound to DNA: Minor groove binding by alpha helices". Science. 266 (5186): 763–70. doi:10.1126/science.7973627. PMID 7973627.
- ↑ Daber, R; Stayrook, S; Rosenberg, A; Lewis, M (2007). "Structural analysis of lac repressor bound to allosteric effectors". J. Mol. Biol. 370: 609–19. doi:10.1016/j.jmb.2007.04.028. PMC 2715899
 . PMID 17543986.
. PMID 17543986. - ↑ Goodsell, D. S. (2003). "Lac Repressor". RCSB Protein Data Bank. doi:10.2210/rcsb_pdb/mom_2003_3.
- ↑ Lewis, M. (Jun 2005). "The lac repressor". Comptes rendus biologies. 328 (6): 521–548. doi:10.1016/j.crvi.2005.04.004. ISSN 1631-0691. PMID 15950160.
- 1 2 Swint-Kruse, L.; Matthews, K. S. (2009). "Allostery in the LacI/GalR Family: Variations on a Theme". Current Opinion in Microbiology. 12 (2): 129–137. doi:10.1016/j.mib.2009.01.009. PMC 2688824
 . PMID 19269243.
. PMID 19269243. - ↑ Oehler, S.; Eismann, E. R.; Krämer, H.; Müller-Hill, B. (1990). "The three operators of the lac operon cooperate in repression". The EMBO Journal. 9 (4): 973–979. PMC 551766
 . PMID 2182324.
. PMID 2182324. - ↑ Gilbert, W; Müller-Hill B (December 1966). "ISOLATION OF THE LAC REPRESSOR". Proc. Natl. Acad. Sci. U.S.A. 56 (6): 1891–1898. doi:10.1073/pnas.56.6.1891. PMC 220206
 . PMID 16591435.
. PMID 16591435.
External links
- Lac Repressors at the US National Library of Medicine Medical Subject Headings (MeSH)
- More information on the lac repressor molecule on protein database
- Lac Repressor in Proteopedia.