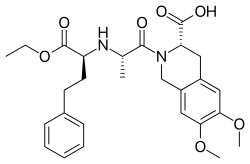
Moexipril
 | |
| Clinical data | |
|---|---|
| Trade names | Univasc |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695018 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | C09AA13 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 13-22% |
| Protein binding | 90% |
| Metabolism | Hepatic (active metabolite, moexiprilat) |
| Biological half-life | 1 hour; 2-9 hours (active metabolite) |
| Excretion | 50% (faeces), 13% (urine) |
| Identifiers | |
| |
| CAS Number |
103775-10-6 |
| PubChem (CID) | 91270 |
| IUPHAR/BPS | 6571 |
| DrugBank |
DB00691 |
| ChemSpider |
82418 |
| UNII |
WT87C52TJZ |
| KEGG |
D08225 |
| ChEMBL |
CHEMBL1165 |
| Chemical and physical data | |
| Formula | C27H34N2O7 |
| Molar mass | 498.568 g/mol |
| | |
Moexipril hydrochloride is a potent orally active nonsulfhydryl angiotensin converting enzyme inhibitor (ACE inhibitor)[1] which is used for the treatment of hypertension and congestive heart failure. Moexipril can be administered alone or with other antihypertensives or diuretics.[2] It works by inhibiting the conversion of angiotensin I to angiotensin II.[3] Moexipril is available from Schwarz Pharma under the trade name Univasc.[3][4]
Pharmacology

Moexipril is available as a prodrug moexipril hydrochloride, and is metabolized in the liver to form the pharmacologically active compound moexiprilat. Formation of moexiprilat is caused by hydrolysis of an ethyl ester group.[5] Moexipril is incompletely absorbed after oral administration, and its bioavailability is low.[6] The long pharmacokinetic half-life and persistent ACE inhibition of moexipril allows once-daily administration.[7]
Moexipril is highly lipophilic,[2] and is in the same hydrophobic range as quinapril, benazepril, and ramipril.[7] Lipophilic ACE inhibitors are able to penetrate membranes more readily, thus tissue ACE may be a target in addition to plasma ACE. A significant reduction in tissue ACE (lung, myocardium, aorta, and kidney) activity has been shown after moexipril use.[8]
It has additional PDE4-inhibiting effects.[9]
Side effects
Moexipril is generally well tolerated in elderly patients with hypertension.[10] Hypotension, dizziness, increased cough, diarrhea, flu syndrome, fatigue, and flushing have been found to affect less than 6% of patients who were prescribed moexipril.[3][10]
Mechanism of action
As an ACE inhibitor, moexipril causes a decrease in ACE. This blocks the conversion of angiotensin I to angiotensin II. Blockage of angiotensin II limits hypertension within the vasculature. Additionally, moexipril has been found to possess cardioprotective properties. Rats given moexipril one week prior to induction of myocardial infarction, displayed decreased infarct size.[11] The cardioprotective effects of ACE inhibitors are mediated through a combination of angiotensin II inhibition and bradykinin proliferation.[8][12] Increased levels of bradykinin stimulate in the production of prostaglandin E2[13] and nitric oxide,[12] which cause vasodilation and continue to exert antiproliferative effects.[8] Inhibition of angiotensin II by moexipril decreases remodeling effects on the cardiovascular system. Indirectly, angiotensin II stimulates of the production of endothelin 1 and 3 (ET1, ET3)[14] and the transforming growth factor beta-1 (TGF-β1),[15] all of which have tissue proliferative effects that are blocked by the actions of moexipril. The antiproliferative effects of moexipril have also been demonstrated by in vitro studies where moexipril inhibits the estrogen-stimulated growth of neonatal cardiac fibroblasts in rats.[12] Other ACE inhibitors have also been found to produce these actions, as well.
Synthesis
The synthesis of the all-important dipeptide-like side chain involves alkylation of the tert-butyl ester of L-alanine (2) with ethyl 2-bromo-4-phenylbutanoate (1); the presominane of the desired isomer is attributable to asymmetric induction from the adjacent chiral center. Reaction of the product with hydrogen chloride then cleaves the tert-butyl group to give the half acid (3).[18] Coupling of that acid to the secondary amine on tetrahydroisoquinoline (4) gives the corresponding amine. The tert-butyl ester in this product is again cleaved with hydrogen chloride to afford moexipril (5).
References
- ↑ Hochadel, Maryanne, ed. (2006). The AARP Guide to Pills. Sterling Publishing Company. p. 640. ISBN 978-1-4027-1740-6. Retrieved 2009-10-09.
- 1 2 Belal, F.F, K.M. Metwaly, and S.M. Amer. "Development of Membrane Electrodes for the Specific Determination of Moexipril Hydrochloride in Dosage Forms and Biological Fluids." Portugaliae Electrochimica Acta. 27.4 (2009): 463-475.
- 1 2 3 Rodgers, Katie, Michael C Vinson, and Marvin W Davis. "Breakthroughs: New drug approvals of 1995 -- part 1." Advanstar Communications, Inc. 140.3 (1996): 84.
- ↑ Dart, Richard C. (2004). Medical toxicology. Lippincott Williams & Wilkins. p. 647. ISBN 978-0-7817-2845-4. Retrieved 2009-10-09.
- ↑ Kalász, H; Petroianu, G; Tekes, K; Klebovich, I; Ludányi, K; Gulyás, Z (2007). "Metabolism of moexipril to moexiprilat: Determination of in vitro metabolism using HPLC-ES-MS". Medicinal chemistry (Shariqah (United Arab Emirates)). 3 (1): 101–106. doi:10.2174/157340607779317490. PMID 17266629.
- ↑ Chrysant, George S, PK Nguyen. “Moexipril and left ventricular hypertrophy.” Vascular Health Risk Management. 3.1 (2007): 23-30.
- 1 2 Cawello, W; Boekens, H; Waitzinger, J; Miller, U (2002). "Moexipril shows a long duration of action related to an extended pharmacokinetic half-life and prolonged ACE inhibition". International journal of clinical pharmacology and therapeutics. 40 (1): 9–17. doi:10.5414/cpp40009. PMID 11837383.
- 1 2 3 Chrysant, S. G. (1998). "Vascular remodeling: The role of angiotensin-converting enzyme inhibitors". American Heart Journal. 135 (2 Pt 2): 21–30. PMID 9488609.
- ↑ Cameron, RT; Coleman, RG; Day, JP; Yalla, KC; Houslay, MD; Adams, DR; Shoichet, BK; Baillie, GS (May 2013). "Chemical informatics uncovers a new role for moexipril as a novel inhibitor of cAMP phosphodiesterase-4 (PDE4)". Biochemical Pharmacology. 85 (9): 1297–1305. doi:10.1016/j.bcp.2013.02.026. PMC 3625111
 . PMID 23473803.
. PMID 23473803. - 1 2 White, W. B.; Stimpel, M (1995). "Long-term safety and efficacy of moexipril alone and in combination with hydrochlorothiazide in elderly patients with hypertension". Journal of human hypertension. 9 (11): 879–884. PMID 8583466.
- ↑ Rosendorff, C (1996). "The renin-angiotensin system and vascular hypertrophy". Journal of the American College of Cardiology. 28 (4): 803–812. doi:10.1016/s0735-1097(96)00251-3. PMID 8837552.
- 1 2 3 Hartman, J.C. “The role of bradykinin and nitric oxide in the cardioprotective action of ACE inhibitors.” The Annals of Thoracic Surgery. 60.3 (1995): 789-792.
- ↑ Jaiswal, N, DI Diz, MC Chappell, MC Khosia, CM Ferrario. “Stimulation of endothelial cell prostaglandin production by angiotensin peptides. Characterization of receptors.” Hypertension. 19.2 (1992): 49-55.
- ↑ Phillips, PA. “Interaction between endothelin and angiotensin II.” Clinical and Experimental Pharmacology and Physiology. 26.7. (1999): 517-518.
- ↑ Youn, T. J.; Kim, H. S.; Oh, B. H. (1999). "Ventricular remodeling and transforming growth factor-beta 1 mRNA expression after nontransmural myocardial infarction in rats: Effects of angiotensin converting enzyme inhibition and angiotensin II type 1 receptor blockade". Basic research in cardiology. 94 (4): 246–253. doi:10.1007/s003950050149. PMID 10505424.
- ↑ M. L. Hoefle, S. Klutchko, EP 49605; eidem, U.S. Patent 4,344,949 (both 1982 to Warner-Lambert).
- ↑ Klutchko, Sylvester; Blankley, C. John; Fleming, Robert W.; Hinkley, Jack M.; Werner, Ann E.; Nordin, Ivan; Holmes, Ann; Hoefle, Milton L.; Cohen, David M.; Essenburg, A. D. (1986). "Synthesis of novel angiotensin converting enzyme inhibitor quinapril and related compounds. A divergence of structure-activity relationships for non-sulfhydryl and sulfhydryl types". Journal of Medicinal Chemistry. 29 (10): 1953–61. doi:10.1021/jm00160a026. PMID 3020249.
- ↑ Kaltenbronn, James S.; Dejohn, Dana; Krolls, Uldis (2009). "SYNTHESIS OF [S-(R∗,R∗)] – ETHYL α–[(1–CARBOXYETHYL) AMINO]–BENEZENEBUTANOATE, AN IMPORTANT INTERMEDIATE IN THE SYNTHESIS OF ANGIOTENSIN CONVERTING ENZYME INHIBITORS". Organic Preparations and Procedures International. 15: 35. doi:10.1080/00304948309355428.
