Amlodipine/valsartan
 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Valsartan | Angiotensin II receptor antagonist |
| Clinical data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | C09DB01 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 1190398-97-0 |
| PubChem (CID) | 11354874 |
| ChemSpider |
13092186 |
| KEGG |
D09745 |
| (verify) | |
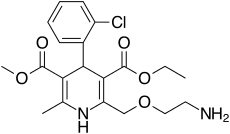
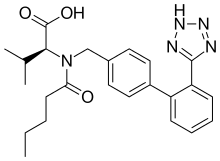
Amlodipine/valsartan is of an oral blood pressure lowering combination drug which combines two medications in a film-coated tablet. It contains amlodipine, a dihydropyridine-type calcium channel blocker, and valsartan, an angiotensin II receptor antagonist (ARB or A2RA); typically formulated as the benzenesulfonate salt. Meta-analysis has shown this combination to be well tolerated and effective for the reduction of blood pressure.[1]
The combination was originally patented by Novartis Pharmaceuticals under the brand name Exforge. The patent expired in September 2012.[2]
Amlodipine/valsartan is available in different dose preparations: 5 mg/80 mg, 5 mg/160 mg, 10 mg/160 mg, 5 mg/320 mg and 10/320 mg of amlodipine and valsartan, respectively.
The combination is also available with included hydrochlorothiazide, for patients who need a three drug regimen to manage their blood pressure.
References
- ↑ Eckert, Siegfried; Freytag, Siegfried B.; Müller, Alfons; Klebs, Sven H. G. (2013). "Meta-analysis of three observational studies of amlodipine/valsartan in hypertensive patients with additional risk factors". Blood Pressure. 22 (sup1): 11–21. doi:10.3109/08037051.2013.793891. ISSN 0803-7051.
- ↑ "Generic Exforge HCT". Blood-pressure.emedtv.com. Retrieved 2012-03-05.