Myotonic dystrophy
| Myotonic dystrophy | |
|---|---|
| Synonyms | dystrophia myotonica, myotonia atrophica, myotonia dystrophica[1] |
|
| |
| 40-year-old with myotonic dystrophy who presented with muscle wasting, bilateral cataracts, and complete heart block | |
| Classification and external resources | |
| Specialty | Medical genetics, pediatrics |
| ICD-10 | G71.1 |
| OMIM | 160900 602668 |
| DiseasesDB | 8739 |
| MeSH | D009223 |
| GeneReviews | |
| Orphanet | 206647 |
Myotonic dystrophy is a long term genetic disorder that affects muscle function. Symptoms include gradually worsening muscle loss and weakness. Muscles often contract and are unable to relax.[1] Other symptoms may include cataracts, intellectual disability, and heart conduction problems.[1][2] In men there may be early balding and an inability to have children.[1]
Myotonic dystrophy is an autosomal-dominant disorder which is typically inherited from a person's parents. There are two main types: type 1 (DM1) due to mutations in the DMPK gene and type 2 (DM2) due to mutations in the CNBP gene. The disorder generally worsens in each generation. A type of DM1 may be apparent at birth. DM2 is generally milder. They are types of muscular dystrophy.[1] Diagnosis is confirmed by genetic testing.[2]
There is no cure.[3] Treatments may include braces or wheelchairs, pacemakers, and non invasive positive pressure ventilation. The medications mexiletine or carbamazepine are occasionally helpful. Pain if it occurs may be treated with tricyclic antidepressants and nonsteroidal anti inflammatory drugs (NSAIDs).[2]
Myotonic dystrophy affects more than 1 in 8,000 people worldwide. While myotonic dystrophy can occur at any age, onset is typically in the 20s and 30s. It is the most common form of muscular dystrophy that begins in adulthood.[1] It was first described in 1909 with the underlying cause of type 1 determined in 1992.[2]
Classification
| Type | Gene | Repeat | Anticipation | Severity |
|---|---|---|---|---|
| DM1 | DMPK | CTG | Yes | Moderate-severe |
| DM2 | ZNF9 | CCTG | Minimal/none | Mild-moderate |
There are two main types of myotonic dystrophy. Type 1 (DM1), also known as Steinert disease, has a severe congenital form and a milder childhood-onset form as well as an adult-onset form.[4] This disease is most often in the facial muscles, levator palpebrae superioris, temporalis, sternocleidomastoids, distal muscles of the forearm, hand intrinsic muscles, and ankle dorsiflexors.[5] Type 2 (DM2), also known as proximal myotonic myopathy (PROMM), is rarer and generally manifests with milder signs and symptoms than DM1.
Other forms of myotonic dystrophy not associated with DM1 or DM2 genetic mutations have been described.[6] One case which was proposed as a candidate for the "DM3" label,[7] was later characterized as an unusual form of inclusion body myopathy associated with Paget's disease and frontotemporal dementia.[6][8][9]
Signs and symptoms
Presentation of symptoms and signs varies considerably by form (DM1/DM2), severity and even unusual DM2 phenotypes. DM1 symptoms for DM2 include problems with executive function (e.g., organization, concentration, word-finding) and hypersomnia. Conduction abnormalities are more common in DM1 than DM2, but all people are advised to have an annual ECG. Both types are also associated with insulin resistance. Myotonic dystrophy may have a cortical cataract with a blue dot appearance, or a posterior subcapsular cataract.[10]
DM2 is generally milder than DM1, with generally fewer DM2 people requiring assistive devices than DM1 people. In addition, the severe congenital form that affects babies in DM1 has not been found in DM2 and the early onset of symptoms is rarely noted to appear in younger people in the medical literature.
Symptoms may appear at any time from infancy to adulthood. DM causes general weakness, usually beginning in the muscles of the hands, feet, neck, or face. It slowly progresses to involve other muscle groups, including the heart. DM affects a wide variety of other organ systems as well.
Genetics
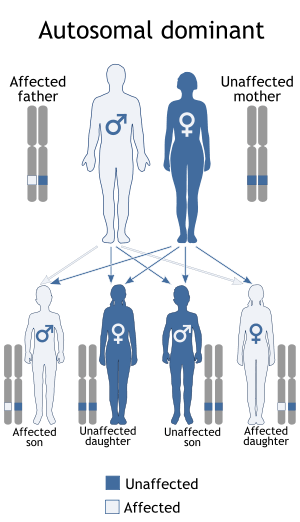
Myotonic dystrophy is a genetic condition which is inherited in an autosomal dominant pattern and thus will be passed along to 50% of a carrier's offspring, on average.Myotonic dystrophy is one of several known trinucleotide repeat disorders. Certain areas of DNA have repeated sequences of two or three nucleotides.
Myotonic dystrophy (DM) is an inherited disease. A severe form of DM, congenital myotonic dystrophy, may appear in newborns of mothers who have DM. Congenital myotonic dystrophy can also be inherited via the paternal gene, although it is said to be relatively rare. Congenital means that the condition is present from birth.
DM1
In DM1, the affected gene is called DMPK, which codes for myotonic dystrophy protein kinase,[11] a protein expressed predominantly in skeletal muscle.[12] The gene is located on the long arm of chromosome 19.[13][14]

In DM1, there is an expansion of the cytosine-thymine-guanine (CTG) triplet repeat in the DMPK gene. Between 5 and 37 repeats is considered normal, while individuals with between 38 and 49 repeats are considered to have a pre-mutation and are at risk of having children with further expanded repeats and, therefore, symptomatic disease.[6] Individuals with greater than 50 repeats are almost invariably symptomatic, with some noted exceptions.[ref] Longer repeats are usually associated with earlier onset and more severe disease.
DMPK alleles with greater than 37 repeats are unstable and additional trinucleotide repeats may be inserted during cell division in mitosis and meiosis. Consequently, the children of individuals with premutations or mutations inherit DMPK alleles which are longer than their parents and therefore are more likely to be affected or display an earlier onset and greater severity of the condition, a phenomenon known as anticipation. Interestingly, paternal transmission of the condition is very uncommon, possibly due to selection pressures against sperm with expanded repeats, but anticipation tends to be less severe than in cases of maternal inheritance.
The RNA from the expanded trinucleotide repeat region forms intranucleoplasmic hairpin loops due to the extensive hydrogen bonding between C-G base pairs, and it has been demonstrated that these sequester the splicing regulator MBNL1 to form distinctive foci by labelling it with GFP and a probe oligonucleotide with the red-fluorescent dye Cyanine5 (Cy5)[15]
DM2
DM2 is caused by a defect of the ZNF9 gene on chromosome 3.[16] The specific defect is a repeat of the cytosine-cytosine-thymine-guanosine (CCTG) tetranucleotide in the ZNF9 gene.[16] As it involves the repeat of four nucleotides, it is not a trinucleotide repeat disorder, but rather a tetranucleotide repeat disorder.[8][17]
The repeat expansion for DM2 is much larger than for DM1, ranging from 75 to over 11,000 repeats.[16] Unlike in DM1, the size of the repeated DNA expansion in DM2 does not appear to make a difference in the age of onset or disease severity.[6] Anticipation appears to be less significant in DM2 and most current reviews only report mild anticipation as a feature of DM2.
Diagnosis
The diagnosis of DM1 and DM2 can be difficult due to the large number of neuromuscular disorders, most of which are very rare. More than 40 neuromuscular disorders exist with close to 100 variants.
As a result, people with multiple symptoms that may be explained by a complex disorder such as DM1 or DM2 will generally be referred by their primary care physician to a neurologist for diagnosis. Depending on the presentation of symptoms, people may be referred to a number of medical specialists including cardiologists, ophthalmologists, endocrinologists, and rheumatologists. In addition, the clinical presentation is obscured by the degree of severity or the presence of unusual phenotypes.
The clinical presentation for both people with DM1 and DM2 commonly differs from the conception of the diseases held by many neurologists. Clinicians who are less familiar with the myotonic dystrophies may expect people with both forms to present with the more severe, classic symptoms of DM1. As a result, people may remain undiagnosed or be misdiagnosed. A useful clinical clue for diagnosis is the failure of spontaneous release of the hands following strong handshakes due to myotonia (delayed relaxation of muscles after contraction) which accompanies muscle weakness.
Though there is presently no cure for DM and management is currently symptom based, a precise diagnosis is still necessary to anticipate multiple other problems that may develop over time (e.g. cataracts). An accurate diagnosis is important to assist with appropriate medical monitoring and management of symptoms. In addition, genetic counseling should be made available to all people because of the high risk of transmission. Potentially serious anesthetic risks are important to note, so the presence of this disorder should be brought to the attention of all medical providers.
Prenatal testing
Genetic tests, including prenatal testing, are available for both confirmed forms. Molecular testing is considered the gold standard of diagnosis.
Testing at pregnancy to determine whether an unborn child is affected is possible if genetic testing in a family has identified a DMPK mutation. This can be done at 10–12 weeks gestation by a procedure called chorionic villus sampling (CVS) that involves removing a tiny piece of the placenta and analyzing DNA from its cells. It can also be done by amniocentesis after 14 weeks gestation by removing a small amount of the amniotic fluid surrounding the baby and analyzing the cells in the fluid. Each of these procedures has a small risk of miscarriage associated with it and those who are interested in learning more should check with their doctor or genetic counselor. There is also another procedure called preimplantation diagnosis that allows a couple to have a child that is unaffected with the genetic condition in their family. This procedure is experimental and not widely available. Those interested in learning more about this procedure should check with their doctor or genetic counselor.
Predictive testing
It is possible to test someone who is at risk for developing DM1 before they are showing symptoms to see whether they inherited an expanded trinucleotide repeat. This is called predictive testing. Predictive testing cannot determine the age of onset that someone will begin to have symptoms, or the course of the disease. If the child is not having symptoms, the testing is not possible with an exception of emancipated minors as a policy.
Management
There is currently no cure for or treatment specific to myotonic dystrophy. Therefore, the focus is on managing the complications of the disease, particularly those relating to the cardiopulmonary system as these account for 70% of deaths due to DM1.[6] Pacemaker insertion may be required for individuals with cardiac conduction abnormalities. Improving the quality of life which can be measured using specific questionnaires[18] is also a main objective of the medical care. Central sleep apnoea or obstructive sleep apnoea may cause excessive daytime sleepiness, and these individuals should undergo a sleep study. Non-invasive ventilation may be offered if there is an abnormality. Otherwise, there is evidence for the use of modafinil as a central nervous system stimulant, although a Cochrane review has described the evidence thus far as inconclusive.
Some small studies have suggested that imipramine, clomipramine and taurine may be useful in the treatment of myotonia.[6] However, due to the weak evidence and potential side effects such as cardiac arrhythmias, these treatments are rarely used. A recent study in December 2015 showed that a common FDA approved antibiotic, Erythromycin reduced myotonia in mice.[19] Human studies are planned for erythromycin. Erythromycin has been used successfully in patients with gastric issues.[20]
Altered splicing of the muscle-specific chloride channel 1 (ClC-1) has been shown to cause the myotonic phenotype of DM1 and is reversible in mouse models using Morpholino antisense to modify splicing of ClC-1 mRNA.[21]
Physical activity
Combined strengthening and aerobic training at moderate intensity was deemed safe for individuals with neuromuscular diseases[22] and the combination was found to increase muscle strength.[23] Specifically, aerobic exercise via stationary bicycle with an ergometer was found to be safe and effective in improving fitness in people with DM1.[24] The strength training or aerobic exercise may promote muscle and cardiorespiratory function, while preventing further disuse atrophy.[22] Cardiovascular impairments and myotonic sensitivities to exercise and temperature necessitate close monitoring of people and educating people in self-monitoring during exercise via the Borg scale, heart rate monitors, and other physical exertion measurements.[25]
Orthotics
Muscular weakness of dorsiflexors (dorsiflexion) hinders the ability to clear the floor during the swing phase of gait and people may adopt a steppage gait pattern[25] or ankle-foot-orthotics may be indicated.[6] Factors such as hand function, skin integrity, and comfort must be assessed prior to prescription. Neck braces can also be prescribed for neck muscle weakness.[6]
Mobility aids and adaptive equipment
Upper and lower limb weakness, visual impairments and myotonia may lead to the need for mobility aids and functional adaptive equipment such as buttonhooks and handled sponges for optimal hand function. If assistive devices and home adaptations are needed, physical therapists may refer on to occupational therapist(s) for further assessment.[6]
Epidemiology
DM1 is the most common form of myotonic muscular dystrophy diagnosed in children, with a prevalence ranging from 1 per 100,000 in Japan to 3-15 per 100,000 in Europe.[6] The prevalence may be as high as 1 in 500 in regions such as Quebec, possibly due to the founder effect. In most populations, DM1 appears to be more common than DM2. However, recent studies suggest that type 2 may be as common as type 1 among people in Germany and Finland.[1]
The incidence of congenital myotonic dystrophy is thought to be about 1:20,000. DM occurs in about 1 per 7,000–8,000 people and has been described in people from all over the world. It affects males and females approximately equally. About 30,000 people in the United States are affected.
Research
The years since the discovery of the genetic cause of MMD in 1992 have been fruitful ones for MMD research. Scientists are gaining understanding of how the expanded DNA section on chromosome 19 causes so many physiologic changes. In the meantime, scientists are also working to test drug treatments that may help symptoms in MMD. Among these are a drug that can make muscles more sensitive to insulin, one that may help improve muscle function and one that may relieve myotonia. The ultimate cure for MMD would probably require finding a way to block the expanded area of DNA on chromosome 19 or chromosome 3 so that it would lose its toxic effect on cells. It is not far-fetched to imagine that, in the future, this expanded section of DNA could be blocked or silenced. Scientists around the world are studying the unusual biological mechanisms that underlie MMD and working on pathways to treatment. However, it needs to be understood that such a treatment will take many years to be sufficiently developed to be used on humans. Ionis Pharmaceutical along with Biogen is currently in Phase 1/2 clinical trials to test an antisense compound to treat Myotonic Dystrophy type 1.
References
- 1 2 3 4 5 6 7 "myotonic dystrophy". GHR. 11 October 2016. Retrieved 16 October 2016.
- 1 2 3 4 Meola, G; Cardani, R (April 2015). "Myotonic dystrophies: An update on clinical aspects, genetic, pathology, and molecular pathomechanisms.". Biochimica et biophysica acta. 1852 (4): 594–606. PMID 24882752.
- ↑ Klein, AF; Dastidar, S; Furling, D; Chuah, MK (2015). "Therapeutic Approaches for Dominant Muscle Diseases: Highlight on Myotonic Dystrophy.". Current gene therapy. 15 (4): 329–37. PMID 26122101.
- ↑ "Juvenile-Onset MMD1". Muscular Dystrophy Association. MDA. Retrieved 17 March 2015.
- ↑ http://www.uptodate.com/contents/myotonic-dystrophy-etiology-clinical-features-and-diagnosis
- 1 2 3 4 5 6 7 8 9 10 Turner, C; Hilton-Jones D. (2010). "The myotonic dystrophies: diagnosis and management". J Neurol Neurosurg Psychiatry. 81 (4): 358–367. doi:10.1136/jnnp.2008.158261. PMID 20176601.
- ↑ Le Ber I, Martinez M, Campion D, et al. (2004). "A non-DM1, non-DM2 multisystem myotonic disorder with frontotemporal dementia: phenotype and suggestive mapping of the DM3 locus to chromosome 15q21-24". Brain. 127 (Pt 9): 1979–92. doi:10.1093/brain/awh216. PMID 15215218.
- 1 2 Dalton, Joline C.; Ranum, Laura PW; Day, John W. (1993-01-01). Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora JH; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C., eds. Myotonic Dystrophy Type 2. Seattle (WA): University of Washington, Seattle. PMID 20301639.updated 2013
- ↑ Udd B, Meola G, Krahe R, et al. (2006). "140th ENMC International Workshop: Myotonic Dystrophy DM2/PROMM and other myotonic dystrophies with guidelines on management". Neuromuscul. Disord. 16 (6): 403–13. doi:10.1016/j.nmd.2006.03.010. PMID 16684600.
- ↑ Yanoff, Myron; Jay S. Duker (2008). Ophthalmology (3rd ed.). Edinburgh: Mosby. p. 411. ISBN 978-0323057516.
- ↑ Mahadevan M, Tsilfidis C, Sabourin L, et al. (March 1992). "Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene". Science. 255 (5049): 1253–5. doi:10.1126/science.1546325. PMID 1546325.
- ↑ van der Ven PF, Jansen G, van Kuppevelt TH, et al. (November 1993). "Myotonic dystrophy kinase is a component of neuromuscular junctions". Human Molecular Genetics. 2 (11): 1889–94. doi:10.1093/hmg/2.11.1889. PMID 8281152.
- ↑ Harley HG, Walsh KV, Rundle S, et al. (May 1991). "Localisation of the myotonic dystrophy locus to 19q13.2-19q13.3 and its relationship to twelve polymorphic loci on 19q". Human Genetics. 87 (1): 73–80. doi:10.1007/BF01213096. PMID 2037285.
- ↑ Bird, Thomas D. (1 January 1993). "Myotonic Dystrophy Type 1". GeneReviews(®). University of Washington, Seattle. Retrieved 9 May 2016.update 2015
- ↑ Ho, Thai H.; Savkur, Rajesh S.; Poulos, Michael G.; Mancini, Michael A.; Swanson, Maurice S.; Cooper, Thomas A. (Jul 1, 2005). "Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy". Journal of Cell Science. 118 (Pt 13): 2923–2933. doi:10.1242/jcs.02404. ISSN 0021-9533. PMID 15961406.
- 1 2 3 Day JW, Ricker K, Jacobsen JF, et al. (February 2003). "Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum". Neurology. 60 (4): 657–64. doi:10.1001/archneur.60.5.657. PMID 12601109.
- ↑ Liquori CL, Ricker K, Moseley ML, et al. (August 2001). "Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9". Science. 293 (5531): 864–7. doi:10.1126/science.1062125. PMID 11486088.
- ↑ Dany, Antoine; Barbe, Coralie; Rapin, Amandine; Réveillère, Christian; Hardouin, Jean-Benoit; Morrone, Isabella; Wolak-Thierry, Aurore; Dramé, Moustapha; Calmus, Arnaud; Sacconi, Sabrina; Bassez, Guillaume; Tiffreau, Vincent; Richard, Isabelle; Gallais, Benjamin; Prigent, Hélène; Taiar, Redha; Jolly, Damien; Novella, Jean-Luc; Boyer, François Constant (2015). "Construction of a Quality of Life Questionnaire for slowly progressive neuromuscular disease". Quality of Life Research. 24 (11): 2615–2623. doi:10.1007/s11136-015-1013-8. ISSN 0962-9343.
- ↑ Masayuki Nakamor1, Katarzyna Taylor, Hideki Mochizuki1, Krzysztof Sobczak, Masanori P. Takahashi1 (2015). "Oral administration of erythromycin decreases RNA toxicity in myotonic dystrophy". Ann Clin Transl Neurol. 3 (1): 42–54. doi:10.1002/acn3.271. PMC 4704483
 . PMID 26783549.
. PMID 26783549. - ↑ Rönnblom A, Andersson S, Hellström PM, Danielsson A (2002). "Gastric emptying in myotonic dystrophy". Eur J Clin Invest. 32 (8): 570–4. doi:10.1046/j.1365-2362.2002.01028.x. PMID 12190956.
- ↑ Wheeler TM, Lueck JD, Swanson MS, Dirksen RT, Thornton CA (2007). "Correction of ClC-1 splicing eliminates chloride channelopathy and myotonia in mouse models of myotonic dystrophy". J. Clin. Invest. 117 (12): 3952–7. doi:10.1172/JCI33355. PMC 2075481
 . PMID 18008009.
. PMID 18008009. - 1 2 Voet NB, van der Kooi EL, Riphagen II, Lindeman E, van Engelen BG, Geurts ACh (2010). Voet, Nicoline BM, ed. "Strength training and aerobic exercise training for muscle disease". Cochrane Database Syst Rev (1): CD003907. doi:10.1002/14651858.CD003907.pub3. PMID 20091552.
- ↑ Cup EH, Pieterse AJ, Ten Broek-Pastoor JM, et al. (November 2007). "Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review". Arch Phys Med Rehabil. 88 (11): 1452–64. doi:10.1016/j.apmr.2007.07.024. PMID 17964887.
- ↑ Orngreen MC, Olsen DB, Vissing J (May 2005). "Aerobic training in patients with myotonic dystrophy type 1". Ann. Neurol. 57 (5): 754–7. doi:10.1002/ana.20460. PMID 15852373.
- 1 2 Pandya, S; Eichinger, K. "Role of physical therapy in the assessment and management of individuals with myotonic dystrophy". Myotonic Dystrophy Foundation. Retrieved 5 May 2012.
Further reading
- Udd, B.; Meola, G.; Krahe, R.; Thornton, C.; Ranum, L.P.W.; Bassez, G.; Kress, W.; Schoser, B.; Moxley, R. (2006-06-01). "140th ENMC International Workshop: Myotonic Dystrophy DM2/PROMM and other myotonic dystrophies with guidelines on management". Neuromuscular Disorders. 16 (6): 403–413. doi:10.1016/j.nmd.2006.03.010. ISSN 0960-8966. PMID 16684600.