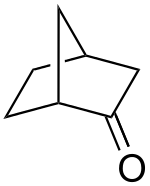
Norcamphor
 | |
| Names | |
|---|---|
| IUPAC name
Bicyclo[2.2.1]heptan-2-one | |
| Other names
2-Norbornanone | |
| Identifiers | |
| 497-38-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 9919 |
| ECHA InfoCard | 100.007.134 |
| PubChem | 10345 |
| |
| |
| Properties | |
| C7H10O | |
| Molar mass | 110.16 g·mol−1 |
| Appearance | colourless solid |
| Melting point | 93 to 96 °C (199 to 205 °F; 366 to 369 K)[1] |
| Boiling point | 168 to 172 °C (334 to 342 °F; 441 to 445 K)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Norcamphor is an organic compound, classified as a bicyclic ketone. It is an analog of camphor, but without the three methyl groups. A colourless solid, it is used as a building block in organic synthesis. Norcamphor is prepared from norbornene via the 2-formate ester, which is oxidized. It is a useful precursor to norborneols.[2]
See also
References
- 1 2 "Norcamphor". Sigma-Aldrich. Retrieved March 11, 2013.
- ↑ Kleinfelter, Donald C.; Schleyer, Paul von R. (1962). "2-Norbornanone". Org. Synth. 42: 79. doi:10.15227/orgsyn.042.0079.
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.