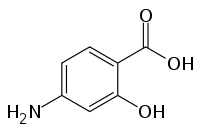
4-Aminosalicylic acid
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Paser |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | J04AA01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 50–60% |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
65-49-6 |
| PubChem (CID) | 4649 |
| DrugBank |
DB00233 |
| ChemSpider |
4488 |
| UNII |
5B2658E0N2 |
| KEGG |
D00162 |
| ChEBI |
CHEBI:27565 |
| ChEMBL |
CHEMBL1169 |
| NIAID ChemDB | 020064 |
| Chemical and physical data | |
| Formula | C7H7NO3 |
| Molar mass | 153.135 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 150.5 °C (302.9 °F) |
| |
| |
| (verify) | |
4-Aminosalicylic acid (para-aminosalicylic acid or PAS) is an antibiotic used to treat tuberculosis.[1] This organic compound has also been use since the 1940s for the treatment of inflammatory bowel diseases (IBDs), where it has shown greater potency in ulcerative colitis and Crohn's disease.[2] It is thought to act via NF-κB (nuclear factor-kappa B) inhibition and free radical scavenging. The closely related chemical compound 5-aminosalicylic acid, sold under the name mesalazine, also has medical uses.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[3]
Medical uses
The main use for 4-aminosalicylic acid is for the treatment of tuberculosis infections.
Tuberculosis
Aminosalicylic acid was introduced to clinical use in 1944. It was the second antibiotic found to be effective in the treatment of tuberculosis, after streptomycin. PAS formed part of the standard treatment for tuberculosis prior to the introduction of rifampicin and pyrazinamide.[4]
Its potency is less than that of the current five first-line drugs (isoniazid, rifampicin, ethambutol, pyrazinamide, and streptomycin) for treating tuberculosis and its cost is higher, but it is still useful in the treatment of multidrug-resistant tuberculosis.[1] PAS is always used in combination with other anti-TB drugs.
The dose when treating tuberculosis is 150 mg/kg/day divided into two to four daily doses; the usual adult dose is therefore approximately 2 to 4 grams four times a day. It is sold in the US as "Paser" by Jacobus Pharmaceutical, which comes in the form of 4 g packets of delayed-release granules. The drug should be taken with acid food or drink (orange, apple or tomato juice).[5] PAS was once available in a combination formula with isoniazid called Pasinah[6] or Pycamisan 33.[7]
The European Medicines Agency (EMA) has recommended granting a marketing authorization for PAS in multidrug-resistant tuberculosis in adults and children when other treatments cannot "be devised for reasons of resistance or tolerability."[8]
Inflammatory bowel disease
PAS has also been used in the treatment of inflammatory bowel disease (ulcerative colitis and Crohn's disease),[2] but has been superseded by other drugs such as sulfasalazine and mesalazine.
Others
PAS has been investigated for the use in manganese chelation therapy, and a 17-year follow-up study shows that it might be superior to other chelation protocols such as EDTA.[9]
Side effects
Gastrointestinal side-effects (nausea, vomiting, diarrhoea) are common; the delayed-release formulation is meant to help overcome this problem.[10] It is also a cause of drug-induced hepatitis. Patients with glucose-6-phosphate dehydrogenase deficiency should avoid taking aminosalicylic acid as it causes haemolysis.[11] Thyroid goitre is also a side-effect because aminosalicylic acid inhibits the synthesis of thyroid hormones.[12]
Drug interactions include elevated phenytoin levels. When taken with rifampicin, the levels of rifampicin in the blood fall by about half.[13]
Pharmacology
With heat, aminosalicylic acid is decarboxylated to produce CO2 and 3-aminophenol.[14]
The U.S. FDA assigned PAS to pregnancy category C, indicating that it is not known whether it will harm an unborn baby.
History
PAS was discovered by the Swedish chemist Jörgen Lehmann upon the report that the tuberculosis bacterium avidly metabolized salicylic acid. Lehmann first tried PAS as an oral TB therapy late in 1944. The first patient made a dramatic recovery.[15] The drug proved better than streptomycin, which had nerve toxicity and to which TB could easily develop resistance. In the 1948, researchers at Britain's Medical Research Council demonstrated that combined treatment with streptomycin and PAS was superior to either drug alone.[1]
Other names
Like many commercially significant compounds, PAS has many names including para-aminosalicylic acid, p-aminosalicylic acid, 4-ASA, and simply P.
Mode of action
PAS has been shown to be a pro-drug and it is incorporated into the folate pathway by dihydropteroate synthase (DHPS) and dihydrofolate synthase (DHFS) to generate a hydroxyl dihydrofolate antimetabolite, which in turn inhibits dihydrofolate reductase (DHFR) enzymatic activity.[16]
References
- 1 2 3 Fox, W.; Ellard, G. A.; Mitchison, D. A. (1999). "Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946-1986, with relevant subsequent publications". The International Journal of Tuberculosis and Lung Disease. 3 (10 Suppl 2): S231–S279. PMID 10529902.
- 1 2 Daniel F, Seksik P, Cacheux W, Jian R, Marteau P (2004). "Tolerance of 4-aminosalicylic acid enemas in patients with inflammatory bowel disease and 5-aminosalicylic-induced acute pancreatitis". Inflamm Bowel Dis. 10 (3): 258–260. doi:10.1097/00054725-200405000-00013. PMID 15290921.
- ↑ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- ↑ Mitchison DA (2000). "Role of individual drugs in the chemotherapy of tuberculosis Role of individual drugs in the chemotherapy of tuberculosis". Int J Tuberc Lung Dis. 4 (9): 796–806. PMID 10985648.
- ↑ "Paser". RxList. Archived from the original on September 13, 2008. Retrieved 2008-10-10.
- ↑ Smith NP, Ryan TJ, Sanderson KV, Sarkany I (1976). "Lichen scrofulosorum: A report of four cases". Br J Dermatol. 94 (3): 319–325. doi:10.1111/j.1365-2133.1976.tb04391.x. PMID 1252363.
- ↑ Black JM; Sutherland, IB (1961). "Two incidents of tuberculous infection by milk from attested herds". Br Med J. 1 (5241): 1732–1735. doi:10.1136/bmj.1.5241.1732. PMC 1954350
 . PMID 20789163.
. PMID 20789163. - ↑ Drug Discovery & Development. EMA Recommends Two New Tuberculosis Treatments. November 22, 2013.
- ↑ Jiang, Y. M.; Mo, X. A.; Du, F. Q.; Fu, X.; Zhu, X. Y.; Gao, H. Y.; Xie, J. L.; Liao, F. L.; Pira, E.; Zheng, W. (2006). "Effective Treatment of Manganese-Induced Occupational Parkinsonism with p-Aminosalicylic Acid: A Case of 17-Year Follow-Up Study". Journal of Occupational and Environmental Medicine. 48 (6): 644–649. doi:10.1097/01.jom.0000204114.01893.3e. PMID 16766929.
- ↑ Das, K. M.; Eastwood, M. A.; McManus, J. P. A.; Sircus, W. (1973). "Adverse Reactions during Salicylazosulfapyridine Therapy and the Relation with Drug Metabolism and Acetylator Phenotype". New England Journal of Medicine. 289 (10): 491–495. doi:10.1056/NEJM197309062891001. PMID 4146729.
- ↑ Szeinberg, A.; Sheba, C.; Hirshorn, N.; Bodonyi, E. (1957). "Studies on erthrocytes in cases with past history of favism and drug-induced acute hemolytic anemia". Blood. 12 (7): 603–613. PMID 13436516.
- ↑ MacGregor, A. G.; Somner, A. R. (1954). "The anti-thyroid action of para-aminosalicylic acid". Lancet. 267 (6845): 931–936. doi:10.1016/S0140-6736(54)92552-0. PMID 13213079.
- ↑ Boman G (1974). "Serum concentration and half-life of rifampicin after simultaneous oral administration of aminosalicylic acid or isoniazid". European journal of clinical pharmacology. 7 (3): 217–25. doi:10.1007/BF00560384. PMID 4854257.
- ↑ Vetuschi, C.; Ragno, G.; Mazzeo, P. (1988). "Determination of p-aminosalicylic acid and m-aminophenol by derivative UV-spectrophotometry". Journal of pharmaceutical and biomedical analysis. 6 (4): 383–391. doi:10.1016/0731-7085(88)80003-7. PMID 16867404.
- ↑ Lehmann, J. (1946). "Para-aminosalicylic acid in the treatment of tuberculosis". Lancet. 1 (6384): 15–16. doi:10.1016/s0140-6736(46)91185-3. PMID 21008766.
- ↑ Zheng, J; Rubin, EJ; Bifani, P; Mathys, V; Lim, V; Au, M; Jang, J; Nam, J; Dick, T; Walker, JR; Pethe, K; Camacho, LR. "para-Aminosalicylic acid is a prodrug targeting dihydrofolate reductase in Mycobacterium tuberculosis". J Biol Chem. 288 (32): 23447–56. doi:10.1074/jbc.m113.475798.